Antifungal Potential of Medicinal Plant Extracts Against Brown Leaf Spot (BLS) Disease of Rice Caused by Bipolaris oryzae
Research Article
Antifungal Potential of Medicinal Plant Extracts Against Brown Leaf Spot (BLS) Disease of Rice Caused by Bipolaris oryzae
Hafiz Muhammad Usama Shaheen1, Nasir Ahmed Rajput1*, Muhammad Atiq1, Ghalib Ayaz Kachelo1, Hadeed Ahmad1, Muhammad Wahab1, Muhammad Faran Tahir1 and Abuzar Hasnain2
1Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan; 2Department of Botany, University of Agriculture, Faisalabad, Pakistan.
Abstract | Rice is susceptible to a number of biotic and abiotic stresses. Among all, brown leaf spot disease of rice caused by Bipolaris oryzae is the most devastating disease, causing potential yield losses. A number of commonly available phytoextracts were explored as potentially safer alternatives to harmful synthetic chemicals for the management of brown leaf spot of rice. For the management of BLS of rice, fourty-four phyto-extracts were screened for their potency in preventing B. oryzae under in-vitro, from where only 8 most effective phyto-extracts were further investigated at different concentrations (5, 10, and 15%) in-vitro using poisoned food technique. From in-vitro experiment, two highly effective extracts were demonstrated for their substantial response against B. oryzae under greenhouse conditions, separately and in combination by using three application methods (Preventive, Curative and after symptom appearance). Findings of contemporary study showed that, Azadirachta indica exhibited the best results in-vitro (10.29 mm) followed by Allium sativum (12.24 mm). Under greenhouse conditions, the combination of Azadirachta indica and Allium sativum expressed significant effect against BLS of rice with least disease incidence percentage (18.07%), when applied as preventive treatment. These extracts could be significant in biological management strategies for the control of BLS of rice, as indicated by the reduction in disease incidence in all experiments as compared to control treatment. The current findings may provide a way for the synthesis and exploitation of new biocontrol agents in the future.
Received | August 21, 2023; Accepted | April 26, 2024; Published | June 05, 2024
*Correspondence | Nasir Ahmed Rajput, Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan; Email: [email protected], [email protected]
Citation | Shaheen, H.M.U., N.A. Rajput, M. Atiq, G.A. Kachelo, H. Ahmad, M. Wahab, M.F. Tahir and A. Hasnain. 2024. Antifungal potential of medicinal plant extracts against brown leaf spot (BLS) disease of rice caused by Bipolaris oryzae. Sarhad Journal of Agriculture, 40(2): 603-614.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.2.603.614
Keywords | Azadirachta indica, Biocontrol, Allium sativum, Poisoned food technique, Synthetic chemicals, Preventive
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Rice (Oryzae sativa L.) belongs to the family Poaceae, is a cereal crop that more than half of the world’s population eats as a staple diet and it is also known as “Queen of cereals” (Rathna et al., 2019; Abbas et al., 2011). It contains a rich amount of starch and delivers more than 20% of the calories that a human body needs (Rout and Tewari, 2012; Pandey, 2015). Rice is cultivating in all continents except Antarctica (Muthayya et al., 2014). Globally, its production was 500 million tones over the area of 167.24 million hectares, while in Pakistan, it was grown on 3.3 million hectares with production of 8.4 million tons (FAO, 2020). The world’s largest producer of rice is China followed by India, Indonesia, Bangladesh, Vietnam, Thailand, Myanmar, Philippines, Japan, and the rest of the world (FAO, 2019). Punjab and Sindh are the major rice-growing provinces of Pakistan and contributing 92% in country’s production (Saqib et al., 2018). Each Pakistani household spends approximately 3.8% of its total food expenditure on rice (Jatoi et al., 2016). Since the year 2000, global rice production has been decreased as compared to consumption (Nguyen, 2002). According to a recent study conducted by the International Food Policy Research Institutes, rice production will need to increase by 38% by 2030 to feed the world’s increasing population, and it will need to be grown on less land as more arable land is lost to housing and industry (Wilson and Talbot, 2009).
Moreover, rice production is extensively affected by several biotic and abiotic factors (Fahad et al., 2019). Numerous bacterial, fungal, viral, and mycoplasmal diseases are reported to affect the crop production. The most significant of these are fungal pathogens such as Pyricularia oryzae (rice blast), Bipolaris oryzae (brown spot), Sclerotium oryzae (stem rot), Rhizoctonia solani (sheath blight) and Sarocladium oryzae (sheath rot). Brown leaf spot of rice caused by the plant pathogenic fungus Bipolaris oryzae, is one of the damaging diseases of rice all over the world (Chouhan and Kumar, 2022). It is a chronic and orphan disease that affects millions of hectares worldwide each year (Zanao et al., 2009). This pathogen was responsible for the Bengal famine in 1942-1943, which resulted in the loss of 50-90% of the rice crop and the starvation deaths of about two million people (Bisen et al., 2015). The pathogen can infect from seedling to maturity stage of plant (Pandey, 2015). Characteristic symptoms of brown leaf spot disease can be seen on both seedlings and mature plants (Imran et al., 2020), firstly, light to dark brown, irregular shaped spots appears on leaf surface. Gray mycelial growth could be visualized between sheaths and stalks and blackening of neck occur in extreme infection (Sunder et al., 2005). Adjacent spots frequently merge to form oval, dark brown lesions that eventually kill the leaves (Aryal et al., 2016) and inadequate grain filling take place under favorable conditions (Huynh and Ashok, 2004). The most favorable environment for brown leaf spot of rice is 25-30℃ temperature and 90% relative humidity for 16-28 hrs (Kumar et al., 2018). The pathogen infects the coleoptiles, causing blighting and turning the leaves oval in shape with dark brown to purplish-brown spots that severely damage photosynthetic activity and eventually kill the leaf. Infected seeds are the primary source of inoculum, whereas secondary infection spread through wind. Weeds and contaminated soil are key sources of pathogen’s survival. Pathogen invades the host plant through stomata and direct penetration (Carvalho et al., 2010).
Excess of nitrogen fertilizer increases the severity of the disease. The rate of disease incidence increases under adverse environmental conditions, causing seed discoloration, decreased seedling vigor, poor grain quality, and yield loss. Various management strategies have been implemented to control the disease such as the use of resistant varieties, cultural, biological and chemical control. No doubt, the use of fungicides is the most effective management option for controlling brown spot-on rice (Goel et al., 2007). However, continuous usage of synthetic chemicals is highly risky for human health and the environment. Chemical residues devastate the soil ecology by damaging non-targeted species, as well as decreasing soil fertility. Now, scientists are keen to find eco-friendly management strategies against plant diseases. Recent studies on phytochemicals in medicinal plants have received a lot of attention, with a focus on their potential to prevent plant diseases (Maninegalai et al., 2011).
It has been demonstrated that using plant-based products and biocontrol agents are both ecologically friendly and efficient against a variety of plant infections. Various plant species have been found to comprise naturally occurring substances that are toxic to many fungi responsible for plant diseases (Schantz et al., 2001). Natural compounds are abundant in the plant world. The majority of higher plants have a wide variety of bioactive plant secondary metabolites (PSMs) that help the plants defend themselves, including phenols, flavonoids, Quinone’s, tannins, alkaloids, saponins, sterols, and terpenoids. Such plant compounds support a variety of biological processes, including antibacterial, allelopathic, antioxidant, and bio-regulatory capabilities of plants. As a result, these natural products can replace dangerous synthetic fungicides for the management of plant diseases (Patel et al., 2013).
Different studies has been reported the usage of phyto-extracts against brown leaf spot of rice. The application of garlic, ginger, sweet flag, onion and aak has been recognized as potentially efficient against B. oryzae under laboratorial environment (Bhattarai et al., 2020). Chouhan and Kumar (2022) have also discussed the antifungal potency of neem (Azadirachta indica), calotropis (Calotropisgigantia), bitter gourd (Momordica charantia), banyan (Ficus bengalensis), Zimmu (Allium cepa L. × Allium sativum L.) and henna (Lawsonia inermis) against B. oryzae.
Therefore, in the light of the above-mentioned significance of brown leaf spot of rice and its management, the current study was conducted to demonstrate the antifungal potency of different medicinal plants as an alternate of synthetic fungicides. Furthermore, this research may aid in a better knowledge of when to apply botanicals at different phases of growth for better effects.
Materials and Methods
Isolation and purification of pathogen
Infected rice samples, collected from surroundings of Faisalabad, were processed for isolation of pathogen on potato dextrose agar (PDA) following standard protocols. Isolated pathogen were purified using single hyphal technique and identified through microscopy. On artificial growth medium, B. oryzae produced cottony dark grey to black to greenish mycelia. Observed microscopic characteristics of B. oryzae were curved, fucoid to cylindrical, light to dark brown spores with septate conidia (Marwein et al., 2022).
In-vitro assessment of phytoextracts against Bipolaris oryzae
Preparation of phytoextracts
Forty-four different plant extracts were evaluated to check their efficacy against the pathogen. Fresh plant materials were collected and brought to the Mycology Laboratory, Department of Plant Pathology, UAF. Plant materials were rinsed with tap water to remove soil debris and then gently washed with the sterilized distilled water. Then, the washed plant parts were left in open air for drying purpose for 3 hours and then put into the brown paper bag and placed them in dry oven. Temperature of dry oven was set at 55°C for duration of 24 hrs to remove excessive moisture. Different plant materials were taken out and grounded into the fine paste (Bulb) and powder (Leaves, seeds, bark and fruits) with the help of electronic blender machine. Flasks (300 ml) were taken, plant material (@10ml/50ml) and ethanol was added into the flask. With the help of aluminum foil, flasks were sealed and put on shaker at 250 rpm for overnight. After that, these mixtures were shaken and filtered into new flasks with four layers muslin cloth. For obtaining the refined extracts, the material was placed on water bath at 55°C temperature for 48 hours.
Screening of phytoextracts against B. oryzae
All the phyto-extracts were examined B. oryzae using the poisoned food technique. To obtain a 10% concentration, 10 ml of aqueous extracts were gently mixed with 90 ml of autoclaved and warmed PDA medium by thorough shaking. A 5 mm fungal plug from one-week old pure culture of B. oryzae was placed in the middle of each plate, after that the plates were wrapped and incubated at 28±2 °C. The control plates were kept untreated. Experiment was arranged following completely randomized design (CRD) with three replications of each treatment. The mycelial growth was regularly observed till the complete mycelial growth of control plate. Finally, the mycelial growth was measured using digital varnier caliper and compared with the control treatment.
In-vitro exploitation of effective phyto-extracts
By following the data of above experiment, eight most effective phyto-extracts namely Azadirachta indica, Allium sativum L, Zingiber officinale, Moringa oleifera, Citrulus calosynthis, Curcuma longa, Datura stramonium and Allium cepa were further investigated against B. oryzae at three concentrations (5, 10 and 15%), respectively. The PDA media was poured in conical flasks; and autoclaved at 121℃ temperature and 15psi pressure for 15-20 minutes. After cooling the media at certain level, 5, 10 and 15ml of prepared phyto-extracts were added in each flask containing 100ml molten PDA media and mixed thoroughly under laminar flow chamber to make concentrations accordingly, where, control treatment was remained untreated. A 5 mm fungal plug from pure culture of B. oryzae was placed in the middle of each plate and incubated at 28±2 °C. The experiment was conducted using completely randomized design (CRD) with three replicates of each treatment. The diameter of the fungus colony was measured after every 24hrs of interval up to 3 days of incubation at 28±2 °C with alternate light and darkness. The mycelial growth was measured using digital varnier caliper.
In-vivo exploitation of phyto-extracts and their combination against brown leaf spot of rice under greenhouse conditions
Nursery of moderately susceptible variety of rice (Shaheen basmati) was grown on raised beds. All the horticultural practices were implemented to grow healthy seedlings. Later on, seedlings of 45 days age were transplanted to 25 cm-diameter earthen pots containing 4 kg sterilized soil. Under a greenhouse condition, three groups of the potted plants were randomly distributed, and all the agronomical practices were ensured to maintain healthy crop. After successful establishment of seedlings, the first group (preventive) was firstly treated with phyto-extracts and then after two days it was artificially inoculated with spore suspension by using hand sprayer. The second group (curative) was inoculated with the spore suspension two days prior to the application of phyto-extracts. The third group of plants were sprayed with phyto-extracts after the disease symptoms became evident (Kachelo et al., 2022). Control plants within each group were similarly inoculated with spore suspension, but instead of being sprayed with phytoextract, they were given sterile distilled water. Disease incidence observations were recorded on weekly basis up to 3 weeks. The data regarding disease incidence percentage was recorded by using following formula:
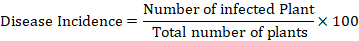
Results and Discussion
Screening of phyto-extracts against B. oryzae under lab conditions
All the phyto-extracts exhibited an inhibitory response against Bipolaris oryzae, however, among all, eight extracts i.e. Azadirachta indica (10.00 mm), Allium sativum L. (11.00 mm), Zingiber officinale (11.58 mm), Moringa oleifera (12.14 mm), Citrulus colocynthis (12.50 mm), Curcuma longa (13.16 mm) Datura stramonium (15.08 mm) and Allium cepa (14.41 mm) exhibited a statistically significant and highly inhibitory response as compared to control plate (59.85 mm) (Table 1), respectively. So, based on these results, eight most effective phyto-extracts were chosen for further investigation in order to assess their efficacy by using different concentrations.
Table 1: In vitro screening of forty four phytoextract @10% concentration against Biploris oryzae.
|
S. No. |
English name |
Botanical name |
Plant parts |
Active ingredients |
Mycelial growth (mm) |
|
1 |
Neem |
Azadirachta indica |
Leaves |
Azadirachtin |
10.00t±0.57 |
|
2 |
Devils Tree |
Alstonia scholaris |
Leaves |
Schloaricine, Alstonine |
24.55mn±1.36 |
|
3 |
Eucalyptus |
Eucalyptus globules |
Leaves |
Flavonoids, Alkaloids, Tannins and Propanoids |
19.16pq±0.88 |
|
4 |
Mint |
Mentha piperita |
Leaves |
Mentha |
35.78efgh±1.12 |
|
5 |
Turmeric |
Curcuma longa |
Fruit |
Monoterpenes |
12.50rst±0.84 |
|
6 |
Black peeper |
Piper nigrum |
Fruit |
Piperine |
20.33opq±1.45 |
|
7 |
Red peeper |
Capsicum annuum |
Fruit |
Capsicine |
23.41mno±3.82 |
|
8 |
Jambolan |
Syzygium cumini |
Seed |
Jambosine |
18.83q±0.44 |
|
9 |
Moringa |
Moringa oleifera |
Leaves |
Moringine |
15.08r±0.72 |
|
10 |
Accacia |
Prosopis juliflora |
Leaves |
Flavonoids |
21.08opq±0.74 |
|
11 |
Lemon |
Citrus limon |
Fruit |
Naringenin |
21.33nopq±0.87 |
|
12 |
Clove |
Syzygium aromaticum |
Fruit |
Eugenol |
19.25pq±0.38 |
|
13 |
Aashoka |
Saraca Asoca |
Leaves |
Catechin |
25.383m±2.68 |
|
14 |
Cactus |
Opuntia ficus-indica |
Leaves |
Flavonoids |
27.93kl±1.17 |
|
15 |
Aloevera |
Aloe vera |
Leaves |
Alkaloids |
19.00q±0.94 |
|
16 |
Cinnamon |
Cinnamomum zeylanicum |
Bark |
Cinnzeylaine |
36.38efg±0.76 |
|
17 |
Mango |
Mangifera indica |
Seed |
Mangiferin |
22.71mno±1.44 |
|
18 |
Brown mustard |
Brassica juncea (L.) Czern |
Seed |
phenolic acids, flavonoids, alkaloids, |
34.46ghi±2.00 |
|
Table continued on next page................ |
|||||
|
S. No. |
English name |
Botanical name |
Plant parts |
Active ingredients |
Mycelial growth (mm) |
|
19 |
Black night shade |
Solanum nigrum L. |
Seeds |
Glycoalkaloids, Polyphenolic compounds |
47.00a±1.04 |
|
20 |
Cardamom |
Amomum subulatum Roxb |
Fruit |
Tannins, Alkaloids and Flavonoids |
43.95ab±0.57 |
|
21 |
Tamarind |
Tamarindus indica L. |
Fruit |
Alkaloid, Phenols, Flavonoid, |
27.50kl±1.32 |
|
22 |
Garlic |
Alliusm sativum L. |
Bulb |
Anthraquinones, Tannin and Terpenoids |
11.00t±0.28 |
|
23 |
Sesame |
Sesamum indicum L. |
Seeds |
Flavonoids, Phenolic acids, Alkaloids, Tannins, Saponins, Steroids |
31.83ij±0.44 |
|
24 |
Myrabolan |
Terminalia arjuna (Roxb. ex DC.) Wight and Arn |
Fruits |
Polyphenols, triterpenoids |
38.46de±0.90 |
|
25 |
Black myrabolan |
Terminalia chebula Retz. |
Fruits |
Glycosides, Alkaloids. |
42.00bc±1.15 |
|
26 |
Caraway |
Carum carvi L. |
Fruits |
Carvone, limonene |
37.93def±1.04 |
|
27 |
Dill |
Anethum graveolens L. |
Fruits |
Carvone |
27.38kl±0.45 |
|
28 |
Hemp |
Cannabis sativa L. |
Leaves |
Phytocannabinoids (pCBs), flavonoids |
34.71fghi±1.39 |
|
29 |
Olive |
Olea europaea |
Leaves |
Oleuropein, Verbascoside, Ligstroside |
40.50cd±0.76 |
|
30 |
Conocarpus |
Conocarpus erectus |
Leaves |
phenolic, flavonoids and tannin |
32.41hij±0.93 |
|
31 |
Parthenium |
Parthenium hysterophorus |
Leaves |
Polyphenols, Alkaloids, Terpenes, Pseudoguaianolides, and Histamines |
33.86ghi±1.11 |
|
32 |
Bitter apple |
Citrulus colocynthis |
Fruit |
Cucurbitacin, Flavonoids, Alkaloids and Phenolic acids, |
12.14rst±0.22 |
|
33 |
Dhatura |
Datura stramonium |
Leaves |
Atropine, Scopolamine and Hyoscymine |
13.16rst±0.28 |
|
34 |
Lemon grass |
Cymbopogon citratus |
Leaves |
Myrcene, Limonene, Citral, Geraniol, Citronellol |
29.11jk±0.80 |
|
35 |
Ginger |
Zingiber officnalis |
Bulb |
Zingiberene |
11.58st±1.12 |
|
36 |
Papaya |
Carica papaya |
Leaves |
Alkaloids (carpaine and pseudocarpaine), Proteolytic enzymes (papain and quimiopapain) |
22.50mnop±0.28 |
|
37 |
Milkweed |
Euphorbia heterophylla |
Leaves |
Saponins, Alkaloids, Flavonoids, Tannins, Phenols |
36.13efg±1.39 |
|
38 |
Night-blooming jasmine |
Cestrum nocturnum |
Leaves |
Flavonoids and Phenolic acids |
36.00efg±0.57 |
|
39 |
Onion |
Allium cepa |
Bulb |
Organosulfur compounds, Phenolic compounds, |
14.41rs±0.46 |
|
40 |
Tobacco |
Nicotiana tabacum |
Leaves |
Flavonoid, tannins, Quinones, Saponins, Steroids, Terrpenoids and Resins |
32.33ij±0.66 |
|
41 |
Tulsi |
Ocimum tenuiflorum |
Leaves |
eugenol, rosmarinic acid, apigenin, myretenal, luteolin, β-sitosterol, and carnosic acid |
37.13defg±1.01 |
|
42 |
Henna tree |
Lawsonia inermis |
Leaves |
Flavanoids, saponins, proteins, alkaloids, terpenoids, quinones, coumarins, xanthones, fat, resin and tannins. |
45.83a±0.44 |
|
43 |
Periwinkle |
Catharanthus roseus |
Leaves |
Alkaloids |
42.16bc±1.01 |
|
44 |
Bakain |
Melia azedarach L |
Leaves |
Alkaloids, Tannins, Saponins, Phenols, glycosides, steroids, terpenoids and flavonoids |
21.21nopq±2.04 |
In vitro screening for antifungal activity (means of mycelial growth ± standard error) of Forty-four phytoextracts @ 10% Concentration by using poisoned food technique. Each mean was calculated from three replicates. Similar alphabets in column are showing that the means are not significantly different.
Results regarding invitro trail expressed that among all the treatments Azadirachta indica was found highly effective against B. oryzae with minimum mycelial growth 10.29 mm followed by Allium sativum L. (12.24 mm), Zingiber officinale (12.84 mm), Citrulus colocynthis (14.28 mm), Datura stramonium (14.83 mm) Curcuma longa (15.35 mm), Allium cepa (17.09 mm) and Moringa oleifera (19.26 mm), in contrast to control treatment (Figure 1). Interaction between treatments and concentrations showed that Azadirachta indica exhibited best results with minimum fungal growth 12.15, 10.1 and 8.63 mm at 5,10 and 15% concentrations respectively, followed by Allium sativum L. (13.6, 12.24 and 10.88 mm), Zingiber officinale (14.06, 12.94 and 11.53 mm), Citrulus colocynthis (15.69, 13.98 and 13.16 mm), Datura stramonium (16.49, 14.46 and 13.55 mm), Curcuma longa (17.34, 14.66 and 14.06 mm), Allium cepa (18.67, 16.26 and 1.34 mm) and Moringa oleifera (21.51, 18.26 and 18.02 mm) as compared to control (Figure 2a).
The purpose of the current tests was to evaluate the effectiveness of various phytoextracts across a range of days. Different phytoextracts showed significant fungicidal activity against the pathogen. With 24-hours of interval, data was recorded for up to three days. With an increase in concentration, a greater inhibition of growth was seen. Interaction of treatments and days showed that Azadirachta indica significantly prevented the mycelial growth (7.73, 10.81 and 12.32 mm) followed by Allium sativum L (9.34, 12.57 and 14.82 mm), Zingiber officinale (9.85, 13.19 and 15.49 mm), Citrulus colocynthis (10.69, 14.66 and 17.47 mm), Datura stramonium (11.05, 15.25 and 18.19 mm), Curcuma longa (11.58, 15.83 and 18.65 mm), Allium cepa (13.32, 16.99 and 20.96 mm), Moringa oleifera (15.6, 19.05 and 23.14 mm) at 1st, 2nd and 3rd day respectively (Figure 2b).
Efficacy of phyto-extracts against brown leaf spot of rice by preventive application
Under greenhouse conditions, the combination of Azadirachta indica and Allium sativum L expressed the maximum efficacy against brown leaf spot of rice, with a notable decrease in disease incidence (18.07%) (P ≤ 0.05), followed by solo application of Azadirachta indica (26.94%) and Allium sativum L (31.50 %), respectively (Figure 3a). The disease incidence percentage was monitored for 21 days with 7-days interval using three treatments i.e. Azadirachta indica, Allium sativum L, and their combination Azadirachta indica and Allium sativum L. Combination of Azadirachta indica and Allium sativum L was found to be most effective with minimum disease incidence (22.08, 17.73 and 14.41%) at 7, 14 and 21 days, followed by Azadirachta indica (34.08, 25.91 and 20.83%) and Allium sativum L (38.16, 31.00 and 25.33%), respectively (Figure 3b).
Efficacy of phyto-extracts against brown leaf spot of rice by curative application
Graph represented that the disease incidence was found to be significantly less in all treated pots over check. In a comparison of treatments and disease incidence, the combination of Azadirachta indica and Allium sativum L exhibited the lowest disease incidence (25.10%), followed by Azadirachta indica (34.17%) and Allium sativum L (34.45%) (Figure 4a). However, Interaction between treatments and days expressed that application of Azadirachta indica and Allium sativum L extract showed lowest disease incidence (29.20, 25.16 and 20.95%) at 1st, and 2nd and 3rd week followed by Azadirachta indica (38.28, 34.66 and 29.58%) and Allium sativum L (42.78, 37.78 and 31.78%) (Figure 4b).
Efficacy of phyto-extracts against brown leaf spot of rice by application after symptom appearance
In this experiment, application of phytoextracts was applied after symptoms became evident. All the treatments showed significant response but less than that of preventive (2 days prior to inoculation) and curative (2 days after inoculation). Graph indicated that combination of Azadirachta indica and Allium sativum L was found to be most effective as compared to Azadirachta indica and Allium sativum L with disease incidence of 29.55%, 40.46% and 36.76%, respectively, (Figure 5a). Impact of treatments and days showed that Azadirachta indica and Allium sativum L was highly effective with least disease incidence of 37, 27.87 23.78% as compared to the solo treatment of Azadirachta indica and Allium sativum L. The efficacy of phyto-extracts was found to be reduced after symptoms became evident (Figure 5b).
Brown leaf spot caused by fungus Bipolaris oryzae, is one of the damaging diseases of rice. There are various methods for controlling the BLS disease, but the instability of the pathogen is a potential threat for the rice crop (Shamim and Singh, 2017). Farmers mostly rely on the vast usage of fungicides, crop rotation, and resistant varieties to manage plant diseases. The natural anti-fungal compounds present in medicinal plants are, nonetheless, effective against plant pathogens and can be a suitable option instead of synthetic chemicals, as these are hazardous to human health and environment (Rajput et al., 2018). The findings of the present study demonstrated the efficacy of most effective phyto-extracts for controlling rice brown spot disease. Natural compounds are abundant in the plant world. The majority of higher plants have a wide variety of bioactive PSMs that help the plants defend themselves, including phenols, flavanoids, Quinone’s, tannins, alkaloids, saponins, sterols, and terpenoids. Such plant compounds support a variety of biological processes, including antibacterial, allelopathic, antioxidant, and bioregulatory capabilities. As a result, these natural products can replace dangerous synthetic fungicides for the management of plant diseases (Patel et al., 2013).
In current study, we looked for potential anti-fungal response in medicinal plants against fungal pathogen Bipolaris oryzae causing the brown leaf spot of rice. Finding of current study revealed that mycelial growth was suppressed by all 44 extracts, whereas, 8 plant extracts showed the most effective antifungal potential, suggesting that phyto-extracts could serve as a dependable source of antifungal defense against B. oryzae. It was very interesting to note that Azadirachta indica was found to be excellent, inhibiting the fungal-growth followed by Allium sativum L, Zingiber officinale, Moringa oleifera, Citrulus calosynthis, Curcuma longa, Datura stramonium, and Allium scepa. Our study is supported by the findings of Al-Hazmi (2013), where neem leaf extract (Azadirachta indica) was highly effective in inhibiting the growth of the Helminthosporium sp. fungi when used in the highest concentration (1:1, v:v). In another experiment, nine phyto-extracts were tested for their ability to inhibit B. oryzae, in which garlic cloves extract inhibited the mycelial growth of fungus by 95.04%, when compared to control (Chouhan and Kumar, 2022). The studeis by Iwuagwu et al. (2020), Nazifa et al. (2021), and Parajuli et al. (2022) also concluded the potency of Azadirachta indica as a potential antifungal agent against different fungal pathogens. Efficacy of garlic (Allium sativum L) has also been examined and found prominent inhibitory activity of garlic against B. oryzae under in-vitro environment. Additionally, it has been studied that different parts of neem plants such as bark, leaf and seeds have different impact on mycelial growth of B. oryzae due to variation of phyto-chemicals in different parts (Chhabra et al., 2023). Basically, neem contains a variety of metabolites such as nimbin, nimbanene, nimbolide, nimbandiol, ascorbic acid and 6-desacetylnimbinene which pose antifungal activity against several fungal pathogens (Ara et al., 1990). Whereas, seeds of neem have azadirachtin as main phyto-constituent which actively affect the growth of microbes (Akhila and Rani, 1999). All the parts of neem can be used to prepare botanical extract, but the seeds have highest potency due to increased level of azadirachtin (Yoon et al., 2013).
In this study, the best performing extracts of Azadirachta indica and Allium sativum L and their combination were further demonstrated under greenhouse conditions where the combination of (A. indica+ A. sativum L) expressed highly significant response against the brown leaf spot of rice with least disease incidence percentage. The contemporary stuidy is in line with Persaud et al. (2022), who assessed the effectiveness of neem extracts against brown leaf spot of rice and obtained significant decline in disease incidence after application of aqueous extract solution at different concentrations. Foliar application of botanical extracts can reduce the disease incidence percentage by influencing the biochemical processes and activating defense enzymes including PAL, TAL, chitinase and glucanase, which activated defense mechanism of plants against brown leaf spot disease (Chhabra et al., 2024). The fungicidal efficacy of neem tree extracts is probably due to these phytochemicals like azadirachtin, and nimbin (Kumar et al., 2017; Saleem et al., 2018).
Conclusions and Recommendations
Our study disclosed the efficacy of Azadirachta indica and Allium sativum L as prominent botanical extracts against brown leaf spot disease of rice by applying solo as well as in combination. This study also concluded that preventive and curative type of applications of botanic extracts are more profitable as compared to disease appearance. It is highly recommended that the combination of neem and garlic can be efficiently used against brown leaf spot of rice, whereas further research on isolation of active ingredients from botanicals with chemical formula and mode of action is the need of hour to minimize the use of hazardous synthetic chemicals against plant diseases.
Acknowledgments
We are highly grateful to Plant Pathogen-Interaction Laboratory at Department of Plant Pathology, University of Agriculture Faisalabad for providing all the resources to accomplish this research.
Novelty Statement
All the selected botanical plant extracts were subjected to contain antifungal potential and can be efficiently used as biological fungicides. Results of the contemporary study may provide a base for researchers to extract the active ingredients among phyto-extracts to prepare biological pesticides.
Author’s Contribution
Hafiz Muhammad Usama Shaheen: Executed the field research and wrote manuscript.
Nasir Ahmed Rajput and Muhammad Atiq: Conceived the idea and supervised the work.
Ghalib Ayaz Kachelo: Reviewed and edited the manuscript.
Muhammad Wahab and Muhammad Faran Tahir: Helped in statistical analysis of the data.
Hadeed Ahmad and Abuzar Hasnain: Helped in conducting experiments and writing the manuscript.
Conflict of interest
The authors have declared no conflict of interest
Refrences
Abbas, A., S. Murtaza, F. Aslam, A. Khawar and S. Rafique. 2011. Effect of processing on nutritional value of rice (Oryza sativa). World J. Med. Sci., 6: 68-73.
Akhila, A. and K. Rani. 1999. Chemistry of the neem tree (Azadirachta indica A. Juss.). Fortschr. Chem. Org. Naturst., 78: 147-149. https://doi.org/10.1007/978-3-7091-6394-8_2
Al-Hazmi, R., 2013. Effect of Neem (Azadirachta indica) leaves and seeds extract on the growth of six of the plant disease causing fungi. Glob. Adv. Microbiol. Res. J., 2(5): 089-098.
Ara, I., S. Faizi and S. Siddiqui. 1990. Tricyclic diterpenoids from root bark of Azadirachta indica. Phytochemistry, 29: 911-914. https://doi.org/10.1016/0031-9422(90)80044-H
Aryal, L., G. Bhattarai, A. Subedi, M. Subedi, B. Subedi and G. Sah. 2016. Response of rice varieties to brown spot disease of rice at Paklihawa Rupandehi. Glob. J. Biol. Agric. Sci., 5(2): 50-54.
Bhattarai, B.R., B.K. Yadav, B. Roshan and S. Chapagain. 2020. In vitro Evaluation of botanical extracts against brown leaf spot (Bipolaris oryzae) of rice at Paklihawa Rupandehi Nepal. Front. Plant Sci., 5: 309-312.
Bisen, K., S. Biswas, V. Kumar, K. Lal, R. Kumar and N. Kumar. 2015. Biochemical changes in relation to brown leaf spot (Drechslera oryzae) resistance in different rice genotypes. J. Plant Stud., 4(2): 81-91. https://doi.org/10.5539/jps.v4n2p81
Carvalho, M.P., F.A. Rodrigues, P.R. Silveira, C.C.L. Andrade, J.C.P. Baroni, H.S. Paye and J.E. Loureiro. 2010. Rice resistance to brown spot mediated by nitrogen and potassium. J. Phytopathol., 158: 160-166. https://doi.org/10.1111/j.1439-0434.2009.01593.x
Chhabra, R., R. Sharma, M.S. Hunjan and P. Sharma. 2024. Foliar spray of botanical extracts influence biochemical processes and plant defence enzymes to ameliorate brown spot induced yield loss in rice. Eur. J. Plant Pathol., 168: 467-483. https://doi.org/10.1007/s10658-023-02776-y
Chhabra, R., R. Sharma, M.S. Hunjan, V.K. Sharma, S. Thakur and S. Chauhan. 2023. Phytochemical characterization and antifungal ability of three meliaceae species against Bipolaris oryzae. Agric. Res. J., 60: 146-152. https://doi.org/10.5958/2395-146X.2023.00023.6
Chouhan, V. and A. Kumar. 2022. Use of phytoextracts and non-conventional chemicals for management of brown leaf spot disease of rice. Pharma. Innov. J., 11: 807-810. https://doi.org/10.2139/ssrn.4005520
Fahad, S., M. Adnan, M. Noor, M. Arif, M. Alam, I.A. Khan, H. Ullah, F. Wahid, I.A. Mian and Y. Jamal. 2019. Major constraints for global rice production. In: Advances in rice research for abiotic stress tolerance. Elsevier Sci., pp. 1-22. https://doi.org/10.1016/B978-0-12-814332-2.00001-0
FAOSTAT Statistical Database, 2019. Food and Agriculture Organization of the United Nations Retrieved from http://www.fao.org/faostat/en/
FAOSTAT Statistical Database, 2020. Food and Agriculture Organization of the United Nations Retrieved from http://www.fao.org/faostat/en/
Faruq, A., M. Amin, M. Islam, M. Islam and M. Alam. 2015. Evaluation of some selected seed treatments against leaf blast, brown spot and narrow brown leaf spot diseases of hybrid rice. Adv. Agric. Biol., 4(1): 8-15. https://doi.org/10.15192/PSCP.AAB.2015.4.1.815
Goel, R., J. Lore and T. Thind. 2007. Performance of different fungicides against multiple diseases of rice. Ind. Pytopathol.,
Harish, S., D. Saravanakumar, R. Radjacommare, E. Ebenezar and K. Seetharaman. 2008. Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. Biol., Contr., 53(3): 555-567. https://doi.org/10.1007/s10526-007-9098-9
Huynh, V.N. and A. Gaur. 2004. Role of Bipolaris oryzae in producing abnormal seedling of rice (Oryzae sativa). Omonrice, 12: 102-108.
Imran, M., S. Sahi, M. Atiq and A. Rasul. 2020. Brown leaf spot: An exacerbated embryonic disease of rice. J. Innov. Sci., 6: 108-125. https://doi.org/10.17582/journal.jis/2020/6.2.108.125
Iwuagwu, C., C. Ononuju, C. Umechuruba, A. Nwogbaga, A. Obidiebube, H. Okolie and A. Uwaoma. 2020. Effect of plant extracts on radial growth of Helminthosporium oryzae causative of brown spot disease of rice under in-vitro. Afr. Crop Sci. J., 28(3): 473-480. https://doi.org/10.4314/acsj.v28i3.10
Janki, K., 2005. Important fungal diseases of rice. Rice in Indian perspective, Part 1 and 2: 963-995.
Jatoi, G.H., M.A. Abro, J.A. Tariq, S. Memon, N. Mangi, M.A. Gadhi, S. Hussain and D. Qiu. 2016. Screening of potential bacterial bio-control agents against the Helminthosporium oryzae in-vitro conditions caused by brown spot of rice. Pak. J. Biotechnol., 13(2): 95-100.
Kachelo, G.A., N.A. Rajput, M. Atiq, S.T. Sahi, N.A. Khan, A. Hameed, N. Muhammad and M.S. Mushtaq. 2022. Antifungal efficacy of plant extracts and chemicals against alternaria leaf spot disease of spinach. Pak. J. Agric. Res., 35(2): 380-387. https://doi.org/10.17582/journal.pjar/2022/35.2.380.387
Kumar, S., N. Prakash and K. Arzoo. 2017. Biological and biotechnological approaches to manage brown spot (Helminthosporium oryzae) disease of rice. Biotic Stress Management in Rice. Acad. Press, pp. 175-203. https://doi.org/10.1201/9781315365534-6
Kumar, S., S. Dwivedi, R. Kumar, A. Dubey, A. Kumar, K. Rao, N. Bhakta and J. Mishra. 2018. Screening of rice germplasm against multiple diseases under drought condition in middle igp of bihar. J. Pharmacog. Phytochem., 1: 3232-3235.
Maninegalai, V., V. Ambikapathy and A. Panneerselvam. 2011. Antifungal potentiality of some medicinal plant extracts against Bipolaris oryzae (Breda de Haan). Asian J. Plant Sci., 1(3): 77-80.
Marwein, R., S. Singh, J. Maharana, S. Kumar, K.P. Arunkumar, N. Velmurugan and C. Chikkaputtaiah. 2022. Transcriptome-wide analysis of north-east indian rice cultivars in response to Bipolaris oryzae infection revealed the importance of early response to the pathogen in suppressing the disease progression. Gene, 809: 146049. https://doi.org/10.1016/j.gene.2021.146049
Muthayya, S., J.D. Sugimoto, S. Montgomery and G.F. Maberly. 2014. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci., 1324: 7-14. https://doi.org/10.1111/nyas.12540
Nazifa, Z., F. Aminuzzaman, L. Laila and M. Rehena. 2021. In vitro efficacy of botanicals against rice blast pathogen Magnaporthe oryzae oryzae. Am. J. Plant Sci., 12(4): 662-678. https://doi.org/10.4236/ajps.2021.124045
Negi, P.S., 2012. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food. Microbiol. 156(1): 7-17. https://doi.org/10.1016/j.ijfoodmicro.2012.03.006
Nguyen, N., 2002. Global climate changes and rice food security. Rome: FAO.
Pandey, S., 2015. Efficacy ofleaf extracts in controlling leaf blast and brown spotin rice (Oryza sativa L.). Int. J. Recent Sci. Res., 6(7): 5476-5479.
Parajuli. M., G.B. Khadka and J. Chaurasia. 2022. A review on comparative effect of chemicals and botanicals in management of brown spot diseases of rice (Oryza sativa L.). Arch. Agric. Environ. Sci., 7(1): 127-131. https://doi.org/10.26832/24566632.2022.0701018
Patel, M.B., S.P. Kumar, N.N. Valand, Y.T. Jasrai and S.K. Menon. 2013. Synthesis and biological evaluation of cationic fullerene quinazolinone conjugates and their binding mode with modeled Mycobacterium tuberculosis hypoxanthine-guanine phosphoribosyltransferase enzyme. J. Mol. Model, 19: 3201-3217. https://doi.org/10.1007/s00894-013-1820-1
Persaud, R., G. Payman, A. Khan, N. Singh, D.A. Persaud and G. Subramanian. 2022. Effectiveness of new fungicides and neem plant aqueous extract against brown spot disease of rice. Int. J. Agric. Policy Res., 11: 1-15. https://doi.org/10.15739/IJAPR.23.001
Rajput, N.A., M. Atiq, N. Javed, Y.H. Ye, Z. Zhao, R.N. Syed, A.M. Lodhi, B. Khan, O. Iqbal and D. Dou. 2018. Antimicrobial effect of Chinese medicinal plant crude extracts against Rhizoctonia solani and Pythium aphanidermatum. Fres. Environ. Bull., 27: 3941-3949.
Rathna, P.T., A.R.L.E. Nelson, K. Ravichandran and U. Antony. 2019. Nutritional and functional properties of coloured rice varieties of south india. J. Ethn. Food, 6: 1-11. https://doi.org/10.1186/s42779-019-0017-3
Rout, S., and S. Tewari. 2012. Amalab-e, a formulated botanical product potential against rice blast incitant Pyricularia grisea. Bioscan, 7(3): 547-552.
Safari, M.M., H.R. Zamanizadeh, G.A. Hejaraude and M. Okhovat. 2006. Identification of the causal agent fungi of rice brown spot disease in Guilan province, J. Agric. Sci. Natl. Resour., 12: 136-145.
Saleem, S., G. Muhammad, M.A. Hussain and S.N.A. Bukhari. 2018. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res., 32(7): 1241-1272. https://doi.org/10.1002/ptr.6076
Saqib, S.E., J.K. Kuwornu, S. Panezia and U. Ali. 2018. Factors determining subsistence farmers’ access to agricultural credit in flood-prone areas of pakistan. Kasetsart J. Soc., 39: 262-268. https://doi.org/10.1016/j.kjss.2017.06.001
Schantz, S.L., D.M. Gasior, E. Polverejan, R.J. McCaffrey, A.M. Sweeney, H. Humphrey and J.C. Gardiner. 2001. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ. Health Perspect., 109(6): 605-611. https://doi.org/10.1289/ehp.01109605
Shamim, M. and K.N. Singh. 2017. Biotic stress management in rice: Molecular approaches: CRC Press. https://doi.org/10.1201/9781315365534
Sharmitha, T., C. Justin and S. Roseleen. 2021. Influence of abiotic factors on stem borer incidence and species distribution in rice (Oryza sativa L.). J. Environ. Biol., 42: 1126-1133. https://doi.org/10.22438/jeb/42/4(SI)/MRN-1533b
Sunder, S., R. Singh, D. Dodan and D. Mehla. 2005. Effect of different nitrogen levels on brown spot (Drechslera oryzae) of rice and its management through host resistance and fungicides. Plant Dis. Res., 20: 111-114.
Vincent, J., 1947. Distortion of fungal hyphae in the presence of certain inhibitors. Natural, 159 (4051): 850-850. https://doi.org/10.1038/159850b0
Vishal, G., S. Naveed, S. Rishu, K. Kavaljeet, S. Ichpal, J. Dolly and K. Atul. 2013. Foliar application of fungicides for the management of brown spot disease in rice (Oryza sativa L.) caused by Bipolaris oryzae. Afr. J. Agric. Res., 8(25): 3303-3309.
Von, B.J. and M.S. Bos. 2005. The changing economics and politics of rice: implications for food security, globalization, and environmental sustainability. IRRI Reports, pp. 7-20.
Webster, R. and P. Gunnell. 1992. Compendium of rice diseases. Am. Phytopathol. Soc. Minneapolis, Arkansas Exp. Stat. Res. Ser., 460(10): 62.
Wilson, R.A. and N.J. Talbot. 2009. Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol., 7(3): 185-195. https://doi.org/10.1038/nrmicro2032
Yoon, M.Y., B. Cha and J.C. Kim. 2013. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J., 29: 1-9. https://doi.org/10.5423/PPJ.RW.05.2012.0072
Zanao, J.L.A., F.A. Rodrigues, R.L.F. Fontes, G.H. Korndo and J.C.L. Neves. 2009. Rice resistance to brown spot mediated by silicon and its interaction with manganese. Phytopathology, 157: 73-78. https://doi.org/10.1111/j.1439-0434.2008.01447.x
To share on other social networks, click on any share button. What are these?







