Antifungal Efficacy of Plant Extracts and Chemicals against Alternaria Leaf Spot Disease of Spinach
Research Article
Antifungal Efficacy of Plant Extracts and Chemicals against Alternaria Leaf Spot Disease of Spinach
Ghalib Ayaz Kachelo1, Nasir Ahmed Rajput1*, Muhammad Atiq1, Shahbaz Talib Sahi1, Nasir Ahmad Khan1, Akhtar Hameed2, Noor Muhammad1 and Muhammad Saqib Mushtaq1
1Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan. 2Institute of Plant Protection, Muhammad Nawaz Sharif University of Agriculture, Multan, Pakistan.
Abstract | To check the efficacy of fungicides and plant extracts against leaf spot disease of spinach caused by Alternaria alternata five plant extracts and five fungicides were evaluated under in vitro and field conditions by using complete randomized design (CRD) and randomized complete block design (RCBD). In plant extracts moringa (Moringa oleifera) showed minimum fungal growth (20.790) mm followed by ginger (Zingiber officinale) (25.910), garlic (Allium sativum) (28.087), neem (Azadirachta indica) (29.010) and aloevera (Aloe barbadensis miller) (29.538) mm as compared to control plates, while in chemicals Tilt showed minimum fungal growth (7.982) followed by Score (10.865), Antracol (11.395), Radomil Gold (11.965) and Blue Copper (14.390) mm as compared to control. In the greenhouse, the most effective fungicide and plant extract were applied as tilt and moringa alone and their combination. The plants treated with tilt + moringa was found effective as compared to alone applications. tilt + moringa showed minimum disease incidence (15%) followed by Tilt (18.33%) and moringa (32.33%) as compared to control. While under the field conditions same combination of Tilt + moringa expressed minimum disease incidence (18%) followed by tilt (20.83%) and moringa (34.50%). By these findings it is recommended to farmers that, Alternaria leaf spot disease of spinach can be managed by using tilt fungicide and moringa extract.
Received | December 14, 2020; Accepted | April 27, 2022; Published | June 28, 2022
*Correspondence | Nasir Ahmed Rajput, Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan; Email: nasir.ahmed@uaf.edu.pk
Citation | Kachelo, G.A., N.A. Rajput, M. Atiq, S.T. Sahi, N.A. Khan, A. Hameed, N. Muhammad and M.S. Mushtaq. 2022. Antifungal efficacy of plant extracts and chemicals against alternaria leaf spot disease of spinach. Pakistan Journal of Agricultural Research, 35(2): 380-387.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.2.380.387
Keywords | Alternaria alternata, Tilt, Moringa, Extracts, Fungicide
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Spinach is one of the most important crops among all vegetables that belongs to family Chenopodiaceae. It is well known due to its taste and presence of essential elements like magnesium, iron, calcium, manganese, folic acid and Vitamins (A, B, C, and E) (Tewani et al., 2016) Spinach play a crucial role in food and medicine industries. It is used as curing agent against constipation, dyspepsia, anemia, neuritis, obesity, bronchitis and high blood pressure (Kumar et al., 2013; Bassey and Khan, 2015)
Spinach crop is susceptible to various bacterial, fungal and viral diseases. Among all fungal diseases Alternaria leaf spot is a potential extortion to the successful production of spinach and causes high yield losses annually (Thomma, 2003). It is believed that genus Alternaria causes heavy losses among all fungal pathogens (Agrios, 2005) and recently it was observed in Saudi Arabia (Marraiki et al., 2012). Characteristic symptoms of spinach leaf spot include irregular, dark brown lesions along with concentric rings. Alternaria causes 32 to 57% yield reduction all over the world. It includes numerous saprophytic and endophytic species which are responsible for heavy yield losses (Mamgain et al., 2013). Among all species of Alternaria the A. alternata, A. tenuissima and A. infectoria are considered to be more harmful (Simmons, 1990).
Huge quantities of chemicals are used to prevent the diseases, but these chemicals left lethal residues which can be very harmful to organisms specially for mankind (Reshu and Khan, 2012). Different management strategies are adopted by the various researchers and farmers towards Alternaria alternata but the most economical and environment friendly is the use of resistant varieties but unfortunately resistant varieties are not always desirable. Spinach leaf spot disease can also be minimized through the application of bio control agents and crop rotation, but when disease appears in epidemic form then farmer need a quick action and quick action can be acquired through the use of fungicides and plant extracts. Extracts of neem (Azadirachta indica) exhibit significant results against bacterial as well as fungal diseases (Hanaa et al., 2011; Gurjar et al., 2012). According to IPM (Integrated Pest Management) fungicides should be applied after the appearance of typical disease symptoms (Wright et al., 2002). High crop quality and best average yield potential can be achieved by spraying 7 to 10 days interval (De Visser, 1998). Therefore, current experiment was designed to evaluate five fungicides (Tilt, Score, Antracol, Radomil Gold and Blue copper) and five botanical extracts (neem, garlic, ginger, moringa, aloe vera) at different concentrations against A. alternata in-vitro conditions to select the most effective fungicide and plant extracts with least toxicity to environment.
Materials and Methods
Isolation, identification and purification of A. alternata
Potato dextrose agar (PDA) medium was used for the isolation of A. alternata and sterilized Petri plates (100mm×15mm) were used during experiment to avoid contamination. Diseased leaves expressing typical symptoms of spinach leaf spot were obtained from experimental area of University of Agriculture, Faisalabad and brought to the laboratory for further processing. Diseased leaves were cut into small pieces (2-3 mm) and sterilized by dipping in 70% ethanol. Diseased parts were placed on Petri plates and then incubated in an incubator (GEN2 BOD). Temperature of the incubator was maintained at 21℃. Plates were observed on a daily basis until grayish white colonies with concentric rings with wavy, fluffy and velvety texture were observed.
In vivo evaluation of botanical extracts against A. alternata
Five plant extracts (neem, garlic, aloevera, ginger, moringa) were evaluated against Alternaria alternata. Leaves of moringa, neem, aloe vera were obtained from UAF while ginger and garlic were collected from a local market Jhang bazaar, Faisalabad.
Ginger, garlic and leaves of moringa, neem and aloevera were washed properly and then ground by adding 50 mL of water in 250g leaves. The materials were grounded separately by using the electric juicer machine. Botanical extracts were filtered through muslin cloth and transferred to plastic bottles. Each bottle was tagged and kept at Room temperature for next 24 hours. Each plant extract was evaluated at two concentrations 15 and 30%. A flask containing media were mixed with solutions of plant extracts one by one. Mixed media was poured into petri plates and inoculated with Alternaria culture. Petri plates were incubated and observed on daily basis. It was recorded that mycelial growth was occurred after 48 hours of incubation.
Following formula was used for determining percent growth inhibition (PGI) (Islam et al., 2004).
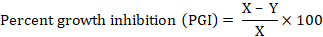
Where; X = Growth of control plate; Y = Growth of the inoculated plate.
In vivo evaluation of fungicides against A. alternata causing leaf spot
Five fungicides (Tilt, Score, Ridomil Gold, Antracol, Blue copper) were evaluated for the management of spinach leaf spot caused by A. alternata through poisoned food technique (Nene and Thapliyal, 1979).
Table 1: List of plant extracts, their botanical names and antimicrobial components.
|
Common name |
Botanical agents |
Authority |
Parts used |
Antimicrobial agents |
|
Neem |
Azadirachta indica |
(A. Juss.) |
Leaves |
Azadirachtin (Pennington et al., 1981) |
|
Moringa |
Moringa oleifera |
(Lam.) |
Leaves |
Caffeoylquinic acid (Anjorin et al., 2010) |
|
Aloe vera |
Aloe barbadensis |
(Miller L. Burm. f. Liliaceae) |
Leaves |
Aloine (Bruneton, 1993) |
|
Garlic |
Allium sativum |
(L.) |
Bulb |
Alicin (Cavallito et al., 1994) |
|
Ginger |
Zingiber officinale |
(Roscoe) |
Rhizome |
Caprylic acid (Ernst and Pittler, 2000) |
Table 2: List of fungicides, their active ingredients and concentrations.
|
Fungicides |
Active ingredients |
Company |
Concentrations used |
|
Tilt |
Propiconazole 25% EC |
Syngenta |
0.08, 0.12 ml/100 ml media |
|
Blue copper |
Copper oxychloride 88% W/W |
Syngenta |
200, 300 mg/100 ml media |
|
Radomil gold |
Mancozeb + Mefenoxam 68% WG |
Syngenta |
200, 300 mg/100 ml media |
|
Score |
Difenoconazole 35% EC |
Syngenta |
0.125, 0.185 ml/100 ml media |
|
Antracol |
Propineb 70% WP |
Bayer crop science |
150, 250 mg/100 ml media |
Potato dextrose media was amended with two different concentrations of each fungicide. With the help of Cork borer 6 mm diameter disc of 7-days old purified fungus colony was transferred to the new PDA plate incorporated with chemicals. The plates were kept for incubation at 25 ± 1°C. PDA plates without chemicals were inoculated and used as control. The fungal colony growth was determined in millimeter. The colony growth was recorded at 24 hours’ interval. Complete randomized design (CRD) was used with five replications of each treatment.
Assessment of fungicide and plant extract against leaf spot of Spinach caused by Alternaria alternata, under greenhouse and field conditions
The available variety of spinach was grown in plastic bags in green house and at field experimental plot at Field area of department of plant pathology, University of agriculture Faisalabad. All the horticultural and cultural practices were applied to grow a healthy field. The best result oriented treatments were used with three replications of each treatment. Moringa extract, tilt fungicide and a mixture of moringa extract and tilt were used at recommended doses for this experiment. Spinach was inoculated by spraying of water based spore suspension. After successful development of disease symptoms, all three treatments were applied. The data was recorded after 7 and 14 days.
Results and Discussion
Evaluation of plant extracts against A. alternata causing leaf spot of spinach
Among all treatments moringa expressed minimum fungal growth (20.790) mm followed by ginger (25.910), garlic (28.087), neem (29.010) and aloe vera (29.538) mm as compared to control plates (Table 3). The impact of treatments and concentrations (T×C) revealed that moringa expressed minimum fungal growth at concentration of 15% (21.604) and 30% (19.814) followed by ginger, garlic, aloe vera and neem at concentration 15% (31.050, 26.081, 31.296 and 30.030) mm and at concentration 30% (19.814, 25.125, 25.740, 27.780 and 27.996) mm as compare to control (Table 4). The interaction between treatments and days (T×D) showed that moringa expressed minimum fungal growth at day 1 (5.583) mm and maximum fungal growth at day 5 (32.133) followed by ginger, neem, aloe vera and garlic shown minimum fungal growth at day 1 (6.083, 6.333, 6.417 and 7.00) mm whereas maximum growth at day 5 (45.967, 45.863, 48.845 and 40.977) mm (Table 5) respectively. Similarly the interaction between treatment, concentration and days expressed that at concentration 15% and day 1 moringa expressed minimum fungal growth (5.833) mm and maximum growth at day 5 (33.318) mm followed by neem, aloe vera, ginger and garlic at concentration 15% and day 1 minimum growth (6.33, 6.50, 7.00 and 7.66) and maximum growth at day 5 (48.063, 50.928, 51.187 and 42.917) mm while at concentration 30% and day 1 moringa expressed minimum growth (5.33) mm and maximum growth at day 5 (30.948) mm, whereas remaining treatments expressed minimum growth at day 1 and concentration 30% (6.3, 6.3, 5.16 and 6.3) mm and maximum growth at day 5 (43.66, 46.76, 40.747 and 39.038) mm respectively as compared to control (Figure 1).
Table 3: Impact of plant extracts on fungal growth under lab conditions.
|
S. No |
Treatments |
Technical name |
Fungal growth (mm) |
|
1 |
Moringa |
Moringa oleifera |
20.709e |
|
2 |
Ginger |
Zingiber officinale |
25.910d |
|
3 |
Garlic |
Allium sativum |
28.087c |
|
4 |
Neem |
Azadirachta indica |
29.010b |
|
5 |
Aloe Vera |
Aloe barbadensis |
29.538b |
|
6 |
Control |
Distilled water |
44.587a |
|
LSD |
0.7101 |
||
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Table 4: Impact of plant extracts and their concentrations on fungal growth under lab conditions.
|
Treatments |
Concentration (%) |
Fungal growth (mm) |
|
Moringa oleifera |
15 |
21.604g |
|
30 |
19.814h |
|
|
Zingiber officinale |
15 |
31.050c |
|
30 |
25.125f |
|
|
Allium sativum |
15 |
26.081f |
|
30 |
25.740f |
|
|
Azadirachta indica |
15 |
31.296c |
|
30 |
27.780c |
|
|
Aloe barbadensis |
15 |
30.030d |
|
30 |
27.996e |
|
|
Control |
43.837b |
|
|
LSD |
0.5507 |
|
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Evaluation of fungicides against A. alternata under lab conditions
Among all fungicides Tilt showed minimum fungal growth (7.982) followed by Score (10.865), Antracol (11.395), Radomil gold (11.965) and Blue copper (14.390) mm as compared to control (Table 6). Impact of treatments and concentrations (T×C) revealed that Tilt expressed minimum fungal growth at concentration of 0.08 mL/100 mL medium (9.435), and 0.12 mL/100 mL medium (6.529) followed by Score at concentration of 0.125 mL/100 mL medium (12.147) and 0.185 mL/100 mL medium (9.584), Antracol at 150 mg/100 mL medium (12.871) and 250 mg/100 mL medium (9.919), Radomil gold at 200 mg/100 mL medium (12.414) and 300 mg/100 mL medium (11.516) and Blue copper at 200 mg/100 mL medium (14.881) and 300 mg/100 mL medium (13.899) mm as compared to the control (Table 7).
Table 5: Impact of Interaction between plant extracts and days on fungal growth under lab conditions.
|
Treatments |
Fungal growth (mm) |
||||
|
Days |
|||||
|
D1 |
D2 |
D3 |
D4 |
D5 |
|
|
Moringa oleifera |
5.583r |
17.626p |
21.714n |
26.489l |
32.133j |
|
Zingiber officinale |
6.083r |
18.965op |
29.573k |
39.849h |
45.967e |
|
Azadirachta indica |
6.333r |
18.268op |
31.840j |
42.744f |
45.863e |
|
Aloe barbadensis |
6.417r |
19.810o |
30.140k |
42.478fg |
48.845d |
|
Allium sativum |
7.000r |
18.295op |
27.965l |
35.318i |
40.977gh |
|
Control |
9.983q |
24.497m |
52.458c |
64.332b |
71.663a |
|
LSD |
1.5879 |
||||
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05). D1= After 24 hours; D2= After 48 hours; D3= After 72 hours; D4= After 98 hours; D5= After 122 hours.
Table 6: Impact of fungicides on fungal growth under lab conditions.
|
S. No. |
Treatments |
Active ingredients |
Fungal growth (mm) |
|
1 |
Tilt |
Propiconazole 25% EC |
7.982f |
|
2 |
Score |
Difenoconazole 35% EC |
10.865e |
|
3 |
Antracol |
Propineb 70% WP |
11.395d |
|
4 |
Radomil gold |
Mancozeb + Mefenoxam 68% WG |
11.965c |
|
5 |
Blue copper |
Copper oxychloride 88% W/W |
14.390b |
|
6 |
Control |
Distilled water |
42.953a |
|
LSD |
0.3894 |
||
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Table 7: Impact of fungicides and their concentrations on fungal growth under lab conditions.
|
Treatments |
Concentrations |
Fungal growth (mm) |
|
Tilt |
0.08mL/100 mL medium |
9.435h |
|
0.12mL/100 mL medium |
6.529i |
|
|
Score |
0.125mL/100 mL medium |
12.147f |
|
0.185 mL/ 100 mL medium |
9.584h |
|
|
Antracol |
150 mg/100 mL medium |
12.871e |
|
250 mg/ 100 mL medium |
9.919h |
|
|
Radomil gold |
200 mg/100 mL medium |
12.414ef |
|
300 mg/100 mL medium |
11.516g |
|
|
Blue copper |
200 mg/100 mL medium |
14.881c |
|
300mg/100 mL medium |
13.899d |
|
|
Control |
Distilled water |
42.548a |
|
LSD |
0.5507 |
|
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Table 8: Impact of fungicides and days on fungal growth under lab conditions.
|
Treatments |
Fungal growth (mm) |
||||
|
Days |
|||||
|
D1 |
D2 |
D3 |
D4 |
D5 |
|
|
Tilt |
0.412s |
3.310p |
9.697l |
11.698k |
14.795i |
|
Blue copper |
1.047rs |
8.185m |
13.267j |
19.902g |
29.550d |
|
Score |
1.348qr |
4.437o |
11.395k |
15.210i |
21.937f |
|
Radomil gold |
1.973q |
6.863n |
9.593l |
17.243h |
24.152e |
|
Antracol |
2.217q |
4.582o |
9.672l |
15.613i |
24.892e |
|
Control |
6.500n |
24.437e |
52.147c |
63.051b |
68.632a |
|
LSD |
0.8708 |
||||
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05). D1= After 24 hours; D2= After 48 hours; D3= After 72 hours; D4= After 98 hours; D5= After 122 hours.
The interaction between treatments and days (T×D) showed that Tilt expressed minimum fungal growth at day 1 (0.412) mm and maximum fungal growth at day 5 (14.795) followed by Blue copper, Score, Radomil gold and Antracol shown minimum fungal growth at day 1 (1.047, 1.348, 1.973 and 2.217 mm) whereas maximum growth at day 5 (29.550, 21.937, 24.152 and 24.89) mm (Table 8), respectively. Similarly the interaction between treatment, concentration and days expressed that at concentration 1 (0.08 mL/100 mL medium) and day 1 Tilt expressed minimum fungal growth (0.71 mm) and maximum growth at day 5 (16.827 mm) followed by Score, Blue copper, Radomil gold and Antracol at concentration 1 and day 1 minimum growth (1.730, 1.117, 2.447 and 2.563) and maximum growth at day 5 (24.683, 30.383, 24.573 and 28.873 mm) while at concentration 2 (0.12 mL/ 100 mL medium) and day 1 Tilt expressed minimum growth (0.113 mm) and maximum growth at day 5 (12.763 mm) whereas remaining treatments Score, Blue copper, Radomil gold and Antracol expressed minimum growth at day 1 and concentration 2 (0.96, 0.977, 1.50, and 1.870 mm) and maximum growth at day 5 (19.190, 28.720, 23.730 and 20.910 mm), respectively as compare to control (Figure 2).
Assessment of fungicide and plant extract against leaf spot of spinach caused by A. alternata under greenhouse conditions
Spinach plants treated with Tilt+Moringa showed minimum disease incidence (15%) followed by Tilt (18.33%) and Moringa (32.33%) as compared to control (Table 9). The interaction between treatments and days (T×D) showed that Tilt+Moringa expressed minimum disease incidence at 10 days (18.33%) followed by Tilt and Moringa at 10 days (22.33 and 35.666%). While after 20 days Tilt+Moringa expressed significant reduction in disease incidence (11.66%), followed by Tilt and Moringa (14.33 and 29%) (Table 10).
Assessment of fungicide and plant extract against A. alternata under field conditions
Among all Tilt+Moringa showed minimum disease incidence (18%) followed by Tilt (20.83%) and Moringa (34.50%) as compared to control (Table 11). The interaction between treatments and days (T×D) showed that Tilt+Moringa expressed minimum disease incidence at 10 days (19.33%) followed by Tilt and Moringa at 10 days (24.00 and 38.33%). While after 20 days Tilt+Moringa expressed significant reduction in disease incidence (16.66%), followed by Tilt and Moringa (17.66 and 30.66%) (Table 12).
Table 9: Impact of treatments on disease incidence under greenhouse conditions.
|
S. No. |
Treatments |
Disease incidence (%) |
|
1 |
Tilt+Moringa oliefera |
15.000c |
|
2 |
Tilt |
18.333c |
|
3 |
Moringa oliefera |
32.333b |
|
4 |
Control |
70.833a |
|
LSD |
3.9886 |
|
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Table 10: Impact of interaction between treatments and days on disease incidence under greenhouse conditions.
|
Treatments |
Disease incidence (%) |
|
|
Days |
||
|
D1 |
D2 |
|
|
Tilt +Moringa oliefera |
18.333ef |
11.667g |
|
Tilt |
22.333e |
14.333fg |
|
Moringa oliefera |
35.667c |
29.000d |
|
Control |
55.000cd |
86.667a |
|
LSD |
5.6407 |
|
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05). *** D1= After seven days; D2= After fourteen days.
Table 11: Impact of treatments on disease incidence under field conditions.
|
S. No. |
Treatments |
Disease incidence (%) |
|
|
1 |
Tilt+Moringa oliefera |
18.00d |
|
|
2 |
Tilt |
20.83c |
|
|
3 |
Moringa oliefera |
34.50b |
|
|
4 |
Control |
80.83a |
|
|
LSD |
2.8155 |
||
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05).
Numerous diseases are the cause of yield reduction in spinach, but the most destructive is the spinach leaf spot. It is a potential threat for the successful cultivation of spinach. When disease appears in epidemic form, then farmers are forced to use something which has a quick action for example botanical extracts and fungicides. For this purpose, in current experiment five botanical extracts (Neem, garlic, aloe vera, moringa, ginger) and five fungicides (Antracol, Radomil gold, Tilt, Blue copper, score) were evaluated against A. alternata. Numerous botanical extracts are mostly preferred against A. alternata because of their least toxicity towards humans and the environment. Among five botanical extracts moringa expressed minimum fungal growth followed by ginger, garlic, neem and aloevera on comparison to control.
Table 12: Impact of interaction between treatments and days on disease incidence under field conditions.
|
Treatments |
Disease incidence (%) |
|
|
Days |
||
|
D1 |
D2 |
|
|
Tilt+Moringa oliefera |
19.33f |
16.66f |
|
Tilt |
24.00e |
17.66f |
|
Moringa oliefera |
38.33c |
30.66d |
|
Control |
68.33c |
93.33a |
|
LSD |
3.9817 |
|
*Mean values with similar letters are not significantly different as determined with LSD test (P≤0.05). D1= After seven days; D2= After fourteen days.
While the interaction between treatments and their concentrations revealed that moringa expressed minimum fungal growth at concentration of 15 and 30%. Outcomes of contemporary study are supported by the work of (Zaffer et al., 2015) who studied the methanolic and ethyl acetate extract of moringa oliefera against Rhizopus stolonifera and Microsporum gypsum and found that ethyl acetate extract significantly inhibited growth of M. gypsum while methanolic extract inhibited R. stolonifera. Results of current experiment are also in line with the findings of (Sharma et al., 2007) who described that neem (Azadirachta indica) extract showed significant inhibition in the radial growth of Alternaria solani by 43.3 and 26.7% at 0.1 and 0.01% concentrations. The results of the present study are favoured by the work of (Ho et al., 2007) who evaluated different botanical extracts and reported that the botanical extracts showed significant reduction in mycelial growth of Alternaria.
No doubt the inappropriate use of chemicals may lead to environmental pollution and health hazards, but when diseases appear in epidemic form, then farmers are bound to use chemicals which are quick in action. For this purpose, in the present study five fungicides were also evaluated at different concentrations to check the fungicides with least toxicity level.
Among all fungicides Tilt showed minimum fungal growth as compared to other fungicides while the interaction between treatments and concentrations revealed that Tilt expressed minimum fungal growth at concentration of 0.08 mL/100 mL media and 0.12 mL/100 mL media. Maximum mycelial growth was observed with the application of Blue copper at 200 mg/100 mL medium and 300 mg/100 mL medium on comparison to the control. The results of the present study are also in agreement with the work of (Sadana and Didwania, 2015), where they applied seven different fungicides against Alternaria solani at three concentrations and got the outstanding results by mancozeb at 1500 ppm concentration. Similarly, (Anwar et al., 2015) also investigated miscellaneous fungicides against Alternaria alternata by using disc plate tactic and found the desirable results. Study of (Kamble et al., 2000) also in in line with current study, where fungicides were evaluated against Alternaria alternata the causal organism of leaf spot of tomato, among all mancozeb was found most effective followed by copper oxychloride and iprodione.
Results of current experiment are in line with the work of (Kumar et al., 2013) who also used Score (fungicide) and reported that it showed significant results against A. solani. Results are also supported by the study of (Valvi et al., 2019) who used seven different fungicides against Alternaria and reported that mancozeb 75% WP significantly inhibited fungal growth followed by propiconazole. Results expressed that mixture of both treatments Tilt + Moringa showed minimum disease incidence (15%) followed by Tilt (18.33%) and Moringa (32.33%) as compared to control, while in field Tilt + Moringa showed minimum disease incidence (18%) followed by Tilt (20.83%) and Moringa (34.50%).
Acknowledgment
Authors are thankful to Phytopathology laboratory, Department of Plant Pathology, University of Agriculture, Faisalabad, to conduct experimental research for M.Sc. studies and Higher Education Commission (HEC) Pakistan for support of funding through HEC Indigenous Scholarship.
Novelty Statement
The novelty of the contemporary research is to assess the efficacy of different phyto-extracts and fungicides against leaf spot of spinach caused by Alternaria alternata. Likewise, this research will also be helpful for farming community to diminish the disease severity/incidence by using the best one of phyto-extracts and fungicide, to enhance their economic return.
Author’s Contribution
GAK: Conducted research and wrote manuscript.
NAR and MA: Conceived idea, gave technical inputs and supervised the research.
STS and NAK: Critically reviewed and edited the manuscript.
AH: Helped in writing manuscript.
NM: Wrote results and discussion section and did necessary corrections.
MSM: Compiled research data.
Conflict of interest
The authors have declared no conflict of interest.
Reference
Agrios, G., 2005. Plant pathology 5th edition: Elsevier academic press. Burlington, Ma. USA. pp. 79-103.
Anjorin, T.S., P. Ikokoh and S. Okolo. 2010. Mineral composition of Moringa oleifera leaves, pods and seeds from two regions in Abuja, Nigeria. Int. J. Agric. Biol., 12(3): 431-434.
Anwar, A., M.S. Haider, M. Aslam, M. Shahbaz, S.N. Khan and A. Bibi. 2015. Assessment of antifungal potentials of some aqueous plant extracts and fungicides against Alternaria alternata. J. Agric. Res., 1: 53.
Bassey, E. and M. Khan. 2015. Proximate composition and phytochemical analysis of bombax buonopozense leaves (Gold coast bombax). Int. J. Curr. Res. Chem. Pharm. Sci., 2(11): 51-56
Bruneton, J., 1993. Pharmacognosie. Phytochimie. Plantes médicinales, Tec. Doc., Paris pp. 363.
Cavallito, C.J., J.S. Buck and C.M. Suter. 1994. Allicin, the antibacterial principle of Allium sativum. Determination of the chemical composition. J. Am. Chem. Soc., 60: 1952-1958.
De Visser, C., 1998. Development of a downy mildew advisory model based on downcast. Eur. J. Plant Pathol., 104: 933-943. https://doi.org/10.1023/A:1008656122629
Ernst, E., and M.H. Pittler. 2000. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br. J. Anaesth., 84(3): 367-371. https://doi.org/10.1093/oxfordjournals.bja.a013442
Gurjar, M.S., S. Ali, M. Akhtar and K.S. Singh. 2012. Efficacy of plant extracts in plant disease management. https://doi.org/10.4236/as.2012.33050
Hanaa, R.F., Z.A. Abdou, D.A. Salama, M.A. Ibrahim and H. Sror. 2011. Effect of neem and willow aqueous extracts on fusarium wilt disease in tomato seedlings: Induction of antioxidant defensive enzymes. Ann. Agric. Sci., 56: 1-7. https://doi.org/10.1016/j.aoas.2011.05.007
Ho, W.C., T.Y. Wu, H.J. Su and W.H. Ko. 2007. Effect of oriental medicinal plant extracts on spore germination of alternaria brassicicola and nature of inhibitory substances from speedweed. Plant Dis., 91: 1621-1624. https://doi.org/10.1094/PDIS-91-12-1621
Islam, M.R., M.K. Hossain, M.H. Bahar and M.R. Ah. 2004. Identification of the causal agent of leaf spot of Betelnut and in vitro evaluation of fungicides and plant extracts against it. Pak. J. Biol. Sci., 7(10): 1758-1761. https://doi.org/10.3923/pjbs.2004.1758.1761
Kamble, P.U., M. Ramiah, and D.V. Patil. 2000. Efficacy of fungicides in controlling leaf spot disease of tomato caused by Alternaria alternata (F.) Kessiler. J. Soils Crops, 10(1): 36-38.
Kumar, S., A. Prasad, S. Iyer and S. Vaidya. 2013. Systematic pharmacognostical, phytochemical and pharmacological review on an ethno medicinal plant, basella alba l. J. Pharm. Phytother., 5: 53-58.
Mamgain, A., R. Roychowdhury and J. Tah. 2013. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 1: 1-9.
Marraiki, N., I. Siddiqui, H. Rizwana and A. Javaid. 2012. First report of Alternaria alternata leaf spots on spinach in saudi arabia. J. Anim. Plant Sci., 22: 247-248.
Nene, Y.L., and P.N. Thapliyal. 1979. Fungicides in plant disease control. 2nd ed. Oxford and IBH pub. Co., New Delhi.
Pennington, T.D., 1981. Flora Neotropica, New York Botanical Garden, NY, 1981, Monogr. No. 28.
Reshu and M.M. Khan. 2012. Role of different microbial-origin bioactive antifungal compounds against Alternaria spp. causing leaf blight of mustard. Plant Pathol. J., 11: 1-9. https://doi.org/10.3923/ppj.2012.1.9
Sadana, D. and N. Didwania. 2015. Bioefficacy of fungi-cides and plant extracts against Alternaria solani causing early blight of tomato. Int. Conf. Plant Mar. Environ. Sci., pp. 1-2.
Sharma, A., A. Dass and M. Paul. 2007. Antifungal effect of neem extract on some common phytopathogenic fungi. Adv. Plant Sci., 20: 357.
Simmons, E.G., 1990. Alternaria themes and variations (27-53). Mycotoxin, 37: 79-119.
Tewani, R., J. Sharma and S. Rao. 2016. Spinach (palak) natural laxative. Int. J. Appl. Res. Tech., 1: 140-148.
Thomma, B.P., 2003. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol., 4: 225-236. https://doi.org/10.1046/j.1364-3703.2003.00173.x
Valvi, H., A. Saykar and V. Bangar. 2019. In vitro and in vivo field efficacy of different fungicides against alternaria brassicae (berk.) sacc. Causing alternaria leaf spot of cauliflower. J. Pharm. Phytochem. 8: 1333-1337. https://doi.org/10.20546/ijcmas.2019.804.223
Wright, D.L., J.J. Marois and J.R. Rich. 2002. Sustainability aspects of precision agriculture for row crops in florida and the southeast us. EDIS, https://doi.org/10.32473/edis-ag186-2002
Zaffer, M., S.A. Ganie, S.S. Gulia, S.S. Yadav, R. Singh and S. Ganguly. 2015. Antifungal efficacy of Moringa oleifera Lam. Am. J. Phytomed. Clin. Ther., 3: 28-33.
To share on other social networks, click on any share button. What are these?







