Allelopathic Potential of Aqueous Extracts of Fresh Leaves of Mucuna bracteata DC and Cymbopogon citratus DC on Weed Germination and Growth
Research Article
Allelopathic Potential of Aqueous Extracts of Fresh Leaves of Mucuna bracteata DC and Cymbopogon citratus DC on Weed Germination and Growth
Siti Fatonah*, Herman and Dewi Yuni Safitri
Department of Biology, Faculty of Mathematics and Natural Science, Universitas Riau, 28293, Kampus Bina Widya Simpang Baru, Pekanbaru, Indonesia.
Abstract | Mucuna bracteata and Cymbopogon citratus are fast-growing plants, easy to find and contain various secondary metabolites. Therefore, these two types of plants can be used as an alternative bioherbicide for weed control. This study aims to determine the effect of aqueous extracts from fresh leaves of C. citratus, M. bracteata and a mixture of both on the germination and growth of Paspalum conjugatum and Borreria alata weeds.Treatments consisted of extracts of M. bracteata, C. citratus and a mixture of both extracts at various concentrations (0% or control, 20% and 60%). Spraying of the extracts started at the time of seed planting, then spraying was done every 3 days for 30 days. All aqueous extract treatments showed toxicity effects on weeds as indicated by the inhibition of germination and growth of P. conjugatum weed and B. alata weed. Mucuna bracteata extract was more effective than C. citratus extract. The mixed extract (C. citratus + M. bracteata) was more effective than the single extract. The highest percentage of germination and growth inhibition was found in the single extract of M. bracteata and mixed extract (M. bracteata + C. citratus) with concentrations of 40% and 60%. Based on the results of this study, 40% fresh extract of M. bracteata or 40% mixed extract of C. citratus + M. bracteata can be applied by spraying directly to the field to inhibit weed emergence and weed growth in agricultural land as an alternative weed control.
Received | February 26, 2024; Accepted | May 16, 2024; Published | June 03, 2024
*Correspondence | Siti Fatonah, Department of Biology, Faculty of Mathematics and Natural Science, Universitas Riau, 28293, Kampus Bina Widya Simpang Baru, Pekanbaru, Indonesia; Email: fath0104@gmail.com
Citation | Fatonah, S., Herman and D.Y. Safitri. 2024. Allelopathic potential of aqueous extracts of fresh leaves of Mucuna bracteata DC and Cymbopogon citratus DC on weed germination and growth. Pakistan Journal of Agricultural Research, 37(2): 115-126.
DOI | https://dx.doi.org/10.17582/journal.pjar/2024/37.2.115.126
Keywords | Cymbopogon citratus, Fresh extract, Germination, Mixed extract, Mucuna bracteata, Weeds
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Weeds are a problem in crop cultivation practices as a result of over-intensive land use. Weeds compete with crops for growing space, light, water, and nutrients, resulting in reduced crop quality, reduced yields, and delays in harvest time (Monteiro and Santos, 2022; Kubiak et al., 2022). Weeds are also hosts for insect pests and pathogens that attack crops and disrupt water flow (Abouziena and Haggag, 2016). About 1800 weed species have caused yield reductions of up to 31.5% (Kubiak et al., 2022).
Common weed controls are physical, mechanical and chemical. Physical and mechanical control includes tillage and hand weeding. Tillage results in increased weed population and soil erosion. Hand weeding is labor intensive and expensive (Sharma and Rayamajhi, 2022). Chemical weed control using herbicides results in contamination of soil, water, air, crops and can kill non-target organisms (Kubiak et al., 2022), reduce farmland biodiversity and loss of natural enemies of crop pests or pollinators (Petit et al., 2011). Chemical herbicides also cause toxicity to animals and plants, which in turn affects human health (Marin-Morales et al., 2013). The various health and environmental problems arising from the use of synthetic herbicides have led many to start implementing organic farming. More sustainable solutions in weed management are being implemented to reduce the impact of herbicide use. One alternative to sustainable weed management is through the phenomenon of allelopathy. Allelopathy is a biological interaction between plants, both inhibiting and promoting growth through the role of chemical compounds produced by plants. Plant allelopathy can be used to control weeds in various ways, such as through ground cover crops, organic mulch, crop rotation, ground cover crops and plant extracts. Plant extracts containing allelochemicals can also be used as bioherbicides. Phytotoxins from plant extracts can be used as alternative bioherbicides to replace herbicides because they are easily decomposed and safe for the environment (Smith-Fiola and Gill, 2014; Abbas et al., 2018; Khamare and Marble, 2022; Scavo and Mauromicale, 2021; Kostina-Bednarz et al., 2023). The phytotoxicity activity of allelochemicals can inhibit weed growth. The use of allelochemicals can be an alternative to reduce herbicide use (Dahiya et al., 2017; Abbas et al., 2021; Kostina-Bednarz et al., 2023).
Various plant extracts containing allelochemicals can inhibit weed germination and growth. Treatment of aqueous extracts from fresh leaves inhibits weed germination and growth, and increases damage to weeds (Fatonah et al., 2014). The treatment of aqueous extracts from dry leaves inhibits weed germination and growth (Sihombing et al., 2012; Taupik et al., 2022; Ismail et al., 2016; Ishak and Sahid, 2014). These studies generally use dry extracts and in single form (without mixture). Dry extracts require large amounts of plant material. One alternative to reduce the need for plant material and increase the toxicity effect of plant extracts is to use plant material in fresh form and in the form of a mixture of extracts from two plants, for example Mucuna bracteata and Cymbopogon citratus. Extracts from fresh plant material (without drying) do not require more plant material and not many chemicals are lost. Mixed extracts allow allelochemicals to have a higher toxicity effect due to the synergistic effect of allelochemicals (Mushtaq et al., 2010; Abbas et al., 2018).
Mucuna bracteata and C. citratus can be a source of bioherbicide. Mucuna bracteata is a ground cover crop that is widely planted in various plantations because it has a high biomass. This plant is resistant to drought and shade, is not favored by livestock, and is able to compete with weeds. Mucuna bracteata contains compounds such as flavonoids, tannins, alkaloids, glycosides, terpenoids, steroids, and saponins (Kumar et al., 2009; Natarajan et al., 2012; Abigail and Dashak, 2020). Cymbopogon citratus or lemongrass is widely found in various regions, has a strong lemon-like aroma, mostly used as spices in food or ingredients for the cosmetics industry. The plant is a producer of alkaloids, saponins, flavonoids, polyphenols, anthraquinones, steroids, and various essential oils (geranial, neral, and myrcene) (Gbenou et al., 2013; Pagare et al., 2015; Oladeji et al., 2019; Gupta et al., 2019). Lemongrass is widely cultivated in Indonesia with a production of about 30-50 tons/ha and can be harvested 3-6 times a year for 4-6 years (Sofiah, 2008).
The effectiveness of the extract in controlling weeds needs to be tested against dominant weeds such as B. alata and P. conjugatum. Borreria alata is a broadleaf weed that easily germinates. Borreria alata and P. conjugatum are dominant weeds in oil palm plantations (Fatonah and Herman, 2011; Dahliani and Elban, 2019; Kurniadie and Umiyati, 2019). Borreria alata is the dominant weed in tomato and chili fields (Abdullah et al., 2020). This study aims to test the phytotoxic effects of aqueous extracts of C. citratus, M. bracteata and a mixture of the two extracts on the germination and growth of P. conjugatum and B. alata weeds.
Materials and Methods
Experimental design
Research in the form of single factor experiment, with a randomized block design. Treatment in the form of extract concentration of M. bracteata and C. Citratus, ie single extract (7 treatments) compared to mixture of extract (three treatments) which can be seen in Table 1. Each treatment was repeated five times. All treatments were tested on both weeds (B. alata and P. conjugatum).
Preparation of extracts, planting seeds of weeds and treatment of extracts
Preparation of extract was carried out by means of fresh leaves of C. citratus and M. bracteata weighed according to treatment (20%, 40%, and 60%) plus water until it reached one liter, then blended. For example, an extract with a concentration of 20% is made by weighing 200 g of plant material plus water until it reaches one liter, then blended and filtered. Seeds of B. alata and P. conjugatum were poured on the soil surface evenly in each polybag. Each polybag contained 20 seeds. The application of M. bracteata and C. citratus extracts were done after seed planting in same day. Extracts were sprayed on soil and weed surfaces every three days for 30 days.
Observation of weed germination and growth
Observations were made daily for 30 days after planting. The variables observed were germination (seedling emergence time, germination percentage, germination inhibition) and growth (fresh weight, number of leaves, shoot length, number of roots, root length, growth inhibition), weed seedling damage, and weed seedling mortality. Inhibition of extract germination shows the effectiveness of the extract in inhibiting weed seed germination with the formula:

Growth inhibition showed the extract effectiveness in inhibiting the growth of weed seedlings with formula:
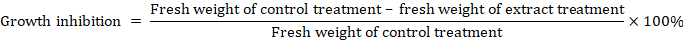
The obtained data were analyzed by using Analysis of Variance (ANOVA). If analysis of variance showed the significant effect, the further test done with Duncan’s Multi Range Test (DMRT) at significance of 5 % by using SPSS 17.
Results and Discussion
Phytotoxic effects of aqueous extracts of M. bracteata DC And C. citratus DC on weed germination
The results of analysis of variance (ANOVA) showed that the treatment of M. bracteata and C. citratus extracts significantly affected the germination of B. alata and P. conjugatum seeds (Table 2). The mean germination time and germination percentage of weeds at various concentrations of M. bracteata and C. citratus extracts are presented in Table 3.
Table 1: Treatment of single and mixture extracts of M. bracteata and C. citratus at various concentrations.
|
Aqueous extracts |
Extract concentration (%) |
Details |
|
Control |
0 |
Air |
|
M. bracteata |
20 |
200 g/l M. bracteata extract |
|
M. bracteata |
40 |
400 g/l M. bracteata extract |
|
M. bracteata |
60 |
600 g/l M. bracteata extract |
|
C. citratus |
20 |
200 g/l of C. citratus extract |
|
C. citratus |
40 |
400 g/l of C. citratus extract |
|
C. citrates |
60 |
600 g/l of C. citratus extract |
|
M. bracteata + C. citratus |
20 |
100 g/l C. citratus extract + 100 g/l M. bracteata extract |
|
M. bracteata + C. citratus |
40 |
200 g/l of C. citratus extract + 200 g/l of M. bracteata extract |
|
M. bracteata + C. citrates |
60 |
300 g/l C. citratus extract+300 g/l M. bracteata extract |
Table 2: ANOVA results of the effect of water extracts of M. bracteata and C. citratus on germination of B. alata and P. conjugatum weeds.
|
Weeds |
Germination parameters |
Sum of squares |
Degrees of freedom |
Mean square |
F-ratio |
Significant (p) |
|
Borreria alata |
Seedling emergence time |
340.171 |
9 |
56.695 |
6.538 |
0.000** |
|
Germination percentage |
24741.520 |
9 |
2749.058 |
141.485 |
0.000** |
|
|
Paspalum conjugatum |
Seedling emergence time |
1128.900 |
9 |
125.433 |
5.295 |
0.000** |
|
Germination percentage |
4208.000 |
9 |
467,556 |
24,289 |
0.000** |
Notes: **: significant at p<0.01
Table 3: Phytotoxic effects of aqueous extracts of M. bracteata and C. citratus on germination of B. alata and P. conjugatum weeds.
|
Aqueous extracts |
Extract concentration (%) |
Germination time (days) |
Germination persentage (%) |
||
|
B. alata |
P. conjugatum |
B. alata |
P. conjugatum |
||
|
Control |
0 |
6.4a |
7.8a |
77c |
32c |
|
M. bracteata |
20 |
11.6b |
9.2a |
5.8ab |
5ab |
|
M. bracteata |
40 |
11b |
30b |
1a |
NGa |
|
M. bracteata |
60 |
30d |
30b |
NG |
NGa |
|
C. citratus |
20 |
10.4b |
8.6a |
8.4b |
10b |
|
C. citratus |
40 |
12.6b |
7.2a |
4.6ab |
6ab |
|
C. citrates |
60 |
13.2b |
8.8a |
5.2ab |
5ab |
|
M. bracteata + C. citratus |
20 |
17.6c |
13.4a |
6.4ab |
4ab |
|
M. bracteata + C. citratus |
40 |
NG |
NG |
NG |
NG |
|
M. bracteata + C. Citrates |
60 |
NG |
NG |
NG |
NG |
Note: The mean number followed by different letters shows a significant difference at the 5% test level based on the DMRT test. NG : seeds do not germinate.
The treatment of M. bracteata and C. citratus extracts slowed down the germination time and decreased the germination percentage. All treatments of C. citratus and M. Bracteata extracts, both single extracts and mixed extracts slowed down the germination time and reduced the germination of B. alata weeds. The 40% treatment of M. Bracteata extract resulted in a very low percentage of Borreria alata weed germination (1%), while the 60% treatment of M. Bracteata extract resulted in B. alata not germinating. The treatment of 20 to 60% C. citratus extract did not slow down the germination time of P. conjugatum weed seeds, but decreased the germination percentage. The treatment of M. bracteata extract, both single extract and mixed extract of M. bracteata + C. citratus at the lowest concentration (20%) did not slow down the germination time, but at higher concentrations (40% and 60%) resulted in P. conjugatum seeds not germinating (0% germination percentage). The mixture of M. bracteata + C. citratus extracts at 40% and 60% concentration resulted in B. alata and P. conjugatum seeds not germinating.
Fresh leaf extracts of M. bracteata and C. citratus showed high effectiveness in inhibiting the germination of weed seeds of B. alata and P. conjugatum with a germination inhibition percentage of 84.37% to 100% (Table 6). The treatment of M. bracteata extract resulted in 100% germination inhibition (no germination) in the 60% concentration treatment for B. alata weed and 40% and 60% concentration treatments for P. conjugatum weed. Germination inhibition of C. Citrates extract on B. alata weeds did not reach 100%, but germination inhibition reached 100% on P. conjugatum weeds (40% and 60% concentration treatments). Germination inhibition from a mixture of M. bracteata + C. citratus extracts reached 100% at 40% and 60% extract concentrations, both on B. alata and P. conjugatum.
The results showed that fresh leaf extracts of M. bracteata and C. citratus inhibited the germination of B. alata and P. conjugatum weed seeds with a high percentage of germination inhibition (84.37% - 100%). Extracts of M. bracteata and C. Citratus inhibit weed germination due to the content of allelochemicals in the extract which results in weeds not germinating because the seeds die or delay germination. Seeds die because allelochemicals cause increased cell permeability and lipid peroxidation, so that the seed embryo is damaged and dies. Another possibility is that the seeds are still alive but delayed in germinating because allelochemicals inhibit the germination process including hydrolysis of food reserves, respiration, and embryo growth. This is related to the effect of allelochemicals in inhibiting water absorption, cell elongation, and cell division, respiration, enzyme activity and function, phytohormone metabolism, and gene expression (Li et al., 2010; Soltys et al., 2013; Khalaj et al., 2013; Feng et al., 2017; Tanase et al., 2019; Bachheti et al., 2020).
The mixed extract of M. bracteata + C. Citratus was more effective in inhibiting germination than the single extract, where B. alata or P. conjugatum weed seeds did not germinate at concentrations of 40 and 60%. The mixed extract of M. bracteata + C. Citratus contains various secondary metabolites, namely a mixture of compounds contained in M. bracteata (flavonoids, tannins, alkaloids, glycosides, terpenoids, steroids, and saponins) (Natarajan et al., 2012; Rane et al., 2019; Abigail and Dashak, 2020) and compounds in C. citrates (alkaloids, saponins, flavonoids, polyphenols, anthraquinones, steroids, and various essential oils) (Gbenou et al., 2013; Oladeji et al., 2019; Gupta et al., 2019). The interaction of these various compounds leads to higher toxicity that inhibits seed germination.
This study used aqueous extracts of fresh leaves of M. bracteata and C. citratus. Fresh leaf extracts require less plant material when compared to dried leaf extracts. Other studies that used aqueous extracts from dried leaves of one plant species showed lower effectiveness in inhibiting weed germination. Water extract from dried leaves of Pueraria javanica with a concentration of 54% showed 53% germination inhibition on Asystasia gangetica weed (Fatonah et al., 2013). Other studies using aqueous extracts from dried leaf leaves generally use lower concentrations, because a little dry matter requires a lot of plant material. A low concentration (6.67%) of aqueous extract from the dried leaves of the legume Leucaena leucocephala inhibited the germination of Emilia sonchifolia weed seeds with a 32% inhibition percentage (Ishak and Sahid, 2014). The aqueous extract of the dried leaves of Jatropha curcas with a concentration of 15% inhibited the germination of Parthenium hyesterophorus seeds with 46% inhibition effectiveness (Khan et al., 2017). However, there are other studies that use aqueous extracts from dried leaves with low concentrations showing high effectiveness in inhibiting weed germination. Water extract from dried leaves of P. javanica at a concentration of 6.67% inhibited the germination of E. indica weed seeds with an inhibitory effectiveness of 95% (Ismail et al., 2016). A 10% aqueous extract of the dried leaves of Chromolaena odorata inhibited the germination of field grass weeds with a 100% inhibition rate (seeds did not germinate) (Suwal et al., 2010). The aqueous extract of the dried leaves of Crotalaria juncea inhibited the germination of Amaranthus hybridus weed with 100% inhibition (no seeds germinated) (Skinner et al., 2012). A 20% aqueous extract of Rhanterium eapposum inhibited the germination of Chenopodiastrum murale weed with up to 100% inhibition effectiveness (Al-Harbi, 2018). A 20% aqueous extract of Datura metel leaves inhibited Parthenium hysterophorus weed germination with 100% inhibitory effectiveness (Ramachandran and Venkataraman, 2016).
Differences in the effectiveness of various extracts in inhibiting the germination of various weeds are related to the content of allelochemicals (plant species), treatment techniques and the level of weed sensitivity to allelochemicals. This study used a mixed treatment of fresh extracts of M. bracteata + C. Citrates on B. alata and P. conjugatum weed seeds grown in soil in polybags. The water extract treatment was carried out by spraying seeds, weeds that have grown and the soil surface evenly at three-day intervals for 30 days. Other studies are generally conducted in the laboratory by planting weed seeds on filter paper in a petri dish, and water extracts are moistened on the seeds and filter paper (Suwal et al., 2010; Sisodia and Siddiqui, 2010; Patil et al., 2013; Woranoot et al., 2015; Al-Harbi, 2018; Taupik et al., 2022). Another study was conducted on soil in polybags, but the extract was done by soaking the seeds in an aqueous extract (Khan et al., 2017). Another study was conducted by extracting various extracts using organic solvents with a low extract concentration (50 mg/l) that could inhibit germination with a high degree of inhibition (Caser et al., 2020). We planted the tested weeds in soil in polybags to illustrate conditions that are almost the same as in the field.
Phytotoxic effects of aqueous extracts of M. bracteata and C. citratus on weed growth
ANOVA results showed that the treatment of M. bracteata and C. citratus extracts significantly affected the growth of B. alata and P. conjugatum weeds (Table 4). The mean fresh weight, number of leaves, plant height, and number of roots in various treatments of M. bracteata and C. citratus extracts are presented in (Table 5).
The results showed that the treatment of C. citratus and M. bracteata extracts reduced the growth of B. alata and P. conjugatum weeds. Growth reduction began at the lowest extract concentration treatment (20%). The 20% treatment of C. citratus extract inhibited fresh weight, plant height, and number of root. The decrease in the number of leaves of P. conjugatum began to occur in the treatment of 20% C. citratus extract, but the decrease in the number of leaves of B. alata began to occur in the treatment of 40% C. citratus extract. The number of leaves of B. alata and P. conjugatum
Table 4: ANOVA results of the effect of water extracts of M. bracteata and C. citratus on growth of B. alata and P. conjugatum weeds.
|
Weeds |
Growth parameters |
Sum of squares |
Degrees of freedom |
Mean square |
F-ratio |
Significant (p) |
|
Borreria alata |
Fresh weight |
4339.171 |
9 |
482.130 |
391.386 |
0.000** |
|
Number of leaves |
866.030 |
9 |
96.226 |
191.770 |
0.000** |
|
|
Shoot length |
368.576 |
9 |
40.953 |
651.660 |
0.000** |
|
|
Number of root |
390.559 |
9 |
43.395 |
128.900 |
||
|
Paspalum conjugatum |
Fresh weight |
2655.383 |
9 |
295.043 |
1780.443 |
0.000** |
|
Number of leaves |
172.583 |
9 |
19.176 |
7.752 |
0.000** |
|
|
Shoot length |
273.120 |
9 |
30.347 |
106.857 |
0.000** |
|
|
Number of root |
337.372 |
9 |
37.486 |
10.167 |
0.000** |
Notes: **: significant at p<0.01
Table 5: Phytotoxic effects of aqueous extracts of M. bracteata and C. citratus on growth of B. alata and P. conjugatum weeds.
|
Plant extract |
Extract concentration (%) |
Fresh weight (mg) |
Number of leaves |
Shoot length (mm) |
Number of root |
||||
|
Ba |
Pc |
Ba |
Pc |
Ba |
Pc |
Ba |
Pc |
||
|
Control |
0 |
3110c |
2452e |
9.36d |
4.88c |
95.3c |
81.5e |
8.02c |
5.46d |
|
M. bracteata |
20 |
40b |
140bc |
7.53b |
2.70b |
12.4b |
9.7cd |
1.14b |
1.05ab |
|
M. bracteata |
40 |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
|
M. bracteata |
60 |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
|
C. citratus |
20 |
110b |
910d |
8.87cd |
4.87c |
15.3b |
16.3d |
2.13c |
2.43c |
|
C. citratus |
40 |
100b |
460bcd |
7.47b |
3.00b |
14.2b |
10.1cd |
1.31b |
1.29bc |
|
C. citrates |
60 |
110b |
660cd |
9.07cd |
2.70b |
15.4b |
10.2cd |
1.36b |
1.87bc |
|
M. bracteata + C. citratus |
20 |
50b |
50b |
8.20bc |
1.80a |
0.90a |
5.1bc |
1.48b |
1.00ab |
|
M. bracteata + C. citratus |
40 |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
|
M. bracteata + C. Citrates |
60 |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
NG |
Note: The mean number followed by different letters shows a significant difference at the 5% test level based on the DMRT test. Ba: B. alata, Pc: P. conjugatum. NG: Seeds do not germinate (weeds do not grow).
weeds in the treatment of M bracteata and C. citratus extracts showed almost the same results as the control treatment and only a slight decrease, but the difference in fresh weight between the extract treatment and the control was higher. This is related to the very small size of leaves and stems in weeds treated with M. bracteata and C. citratus extracts which can be seen in Figures 1 and 2. The treatment of a single extract of M. bracteata and a mixture of extracts of M. bracteata + C. citratus reduced growth starting at a concentration of 20%. The growth appearance of P. conjugatum and B. alata weeds is presented in Figures 1 and 2. Fresh leaf extracts of M. bracteata and C. citratus showed high effectiveness in inhibiting the growth of B. alata and P. conjugatum weeds with a growth inhibition percentage of 96% to 100% (Table 6). This can be seen from the very small size of the weeds (Figures 1 and 2).
Research on the use of plant extracts to inhibit weed growth generally uses leaf extracts or other parts that have been dried. Dry extract treatment with low concentrations generally inhibits weed growth. Treatment of 18% dry extract of Calopogonium mucunoides inhibited the growth of Asystasia gangetica in polybags with a decrease in growth (fresh weight) of 93% (Sihombing et al., 2012). Treatment of 6.67% dry leaf extract of Leucaena leucocephala on Ageratum conyzoides in petridish reduced growth with a percentage reduction of 50% (Ishak and Sahid, 2014). Biological test results of 6.67% dry extract of Pueraria javanica leaves on Chromolaena odorata weeds reduced growth by 97% (Ismail et al., 2016). Treatment of 2.5% dry extract of Parthenium hysterophorus leaves reduced the hypocotyl length of weeds Digitaria sanguinalis and Eleusine indica with an inhibition percentage of 100% (Bashar et al., 2022). Treatment of 10% dry extract of Chromolaena odorata leaves reduced the shoot height of barnyard grass with a decrease of 91.64% (Suwal et al., 2010). The treatment of dried leaf extracts from various plant species inhibited weed growth at lower extract concentrations than fresh leaf extracts of M. bracteata and C. citratus. However, the dry extract in its application requires more plant material than the fresh extract.
The results of this study showed that the treatment of water extracts from fresh leaves of M. bracteata and C. citratus, both single extracts and mixed extracts showed high growth inhibition effectiveness on B. alata and P. conjugatum weeds. The effectiveness of growth inhibition reached 96.28 to 100% ranging from the lowest concentration (20%) to the highest concentration (60%). Research on the use of plant extracts to inhibit weed growth generally uses leaf extracts or other parts that have been dried. Treatment of dried leaf extracts from various plant species inhibited weed growth at lower extract concentrations than fresh leaf extracts of M. bracteata and C. citratus. However, dry extracts in their application required more plant material than fresh extracts. The treatment of 18% dry extract of C. mucunoides inhibited the growth of A. gangetica in polybags with a 93% reduction in growth (fresh weight) (Sihombing et al., 2012). Treatment of 6.67% dry leaf extract of Leucaena leucocephala on Ageratum conyzoides in petridish reduced growth with a percentage decrease of 50% (Ishak and Sahid, 2014). Biological test results of 6.67% dry extract of P. javanica leaves on C. odorata weeds reduce growth by 97% (Ismail et al., 2016). The treatment of 2.5% dry extract of Parthenium hysterophorus leaves reduces the hypocotyl length of Digitaria sanguinalis and Eleusine indica weeds with a percentage inhibition of 100% (Bashar et al., 2022). The treatment of 10% dry extract of C. odorata leaves reduced the shoot height of barnyard grass with a decrease of 91.64% (Suwal et al., 2010).
The results showed that mixed extracts from two plants (M. bracteata + C. citratus) were more effective than single extracts (from one type of plant). This was indicated by lower growth parameter values and higher percentage of growth inhibition in weeds treated with mixed extracts (Tables 5 and 6). This is because the mixed extracts from two types of plants contain more types of secondary metabolites and synergize in inhibiting growth. These results are supported by other studies which also show that mixed extracts of several plants are more effective in inhibiting weed germination and growth. Water extracts from a mixture of two types of plants (Sorghum + sunflower) or more (Sorghum + sunflower + Brassica; Sorghum + sunflower + Brassica + mulberry) were more effective than extracts from one type of plant in inhibiting germination and growth of Trianthema portulacastrum weeds (Mushtaq et al., 2010).
Table 6: Inhibition of aqueous extracts of M. bracteata and C. citratus on germination and growth of weeds.
|
Extracts |
Extract concentration (%) |
Germination inhibition (%) |
Growth inhibition (%) |
||
|
B. alata |
P. conjugatum |
B. alata |
P. conjugatum |
||
|
Control |
0 |
0 |
0 |
0 |
0 |
|
M. bracteata |
20 |
92.46 |
84.37 |
99.85 |
99.41 |
|
M. bracteata |
40 |
98.7 |
100 |
100 |
100 |
|
M. bracteata |
60 |
100 |
100 |
100 |
100 |
|
C. citratus |
20 |
89,09 |
84.37 |
99.64 |
96.28 |
|
C. citratus |
40 |
94.02 |
100 |
99.72 |
98.10 |
|
C. citrates |
60 |
93.24 |
100 |
99.65 |
97.29 |
|
M. bracteata + C. citratus |
20 |
91.68 |
87.5 |
99.84 |
99.79 |
|
M. bracteata + C. citratus |
40 |
100 |
100 |
100 |
100 |
|
M. bracteata + C. Citrates |
60 |
100 |
100 |
100 |
100 |
The growth of B. alata and P. conjugatum weeds is inhibited due to the entry of allelochemicals from C. citratus and M. bracteata extracts into plant tissues, starting from ungerminated seeds to weed seedlings. The effect of allelochemicals on weed germination then affects weed growth. The germination time in the extract treatment was slower than the control, which was 5 to 10 days for B. alata weed and 1 to 6 days for P. conjugatum weed. The slow germination resulted in lower weed growth. Another possibility is that the extracts of C. citratus and M. bracteata applied directly on the weeds have a direct effect on various growth processes. Various allelochemicals inhibit photosynthesis, respiration, and ATP synthesis (Hussain and Reigosa, 2011; Cheng and Cheng, 2015; Soltys et al., 2013; Latif et al., 2017), inhibit water and nutrient uptake, oxidative stress (Ogunsusi et al., 2018; Novakoski et al., 2020; Staszek et al., 2021) and alter cell permeability and membrane function (Omezzine et al., 2014; Latif et al., 2017; Staszek et al., 2021).
The treatment of C. citratus and M. bracteata extracts singly or in mixture inhibited the germination and growth of P. conjugatum and B. alata. The extracts also caused the death of seedlings, but death only occurred at 40% concentration for M. bracteata, with 100% mortality rate for B. alata. The death of B. alata weed occurred in the third week, with all the damage that appeared since the second week such as leaves turning red, yellowing leaves, brown spots, wilting, stems turning brown and gradually dying. The results showed that B. alata weed was more sensitive than P. conjugatum. This shows that dicotyledonous weeds (broadleaf weeds) are more sensitive than monocotyledonous weeds (narrow-leaved weeds). The death of B. alata is caused by allelochemicals contained in M. bracteata extract which are toxic to plants, so they can cause death. Allelochemicals inhibit photosynthesis, respiration, and are able to bind proteins (Soltys et al., 2013). M. bracteata is classified as Leguminosae which generally contains flavonoids which are included in the phenol group. Allelochemicals increase cell membrane permeability, result in solute leakage, and increase lipid peroxidation (Li et al., 2018; Ni et al., 2018; M'barek et al., 2019; Zhang et al., 2020). As a result, growth is inhibited or plant tissues die. Allelochemicals also inhibit nutrient uptake resulting in abnormal growth. Allelochemicals inhibit root elongation, cell division, and the formation of cell ultrastructure, thereby disrupting plant growth and development (Zeng et al., 2001; Li, 2010; Cheng and Cheng, 2015; Zhang et al., 2020).
A single 40% extract of M. bracteata or a mixed extract of C. citratus + M. bracteata was more effective in inhibiting weed germination and growth than a single extract of C. citratus. Important information from this study is that a single extract of 40% M. bracteata or a mixed extract of C. citratus + M. bracteata can be applied by spraying the extract directly on the soil, stem surface and leaves of weed seedlings. Weed germination inhibition is important in weed management as it reduces weed infestation in agricultural fields. This is important for controlling annual weeds or weeds that reproduce by using seeds, generally dicotyledonous weeds or broadleaf weeds (Sharma et al., 2020; Travlos et al., 2020; Bekuzarova et al., 2020; Farooq et al., 2020; Kubiak et al., 2022). Weed growth inhibition is important in weed management through its effect in increasing crop-weed competition, reducing weed regeneration and decreasing weed seed bank (Rao and Matsumoto, 2017; Jha et al., 2017; Farooq et al., 2020).
Conclusions and Recommendations
The treatment of mixed extracts (C. citratus + M. bracteata) was more effective than single extracts in inhibiting the germination and growth of P. conjugatum and B. alata weeds. The highest percentage of germination and growth inhibition was found in the single extract of M. bracteata and mixed extract (C. citratus + M. bracteata) at 40% and 60% concentrations. Based on the results of this study, 40% extract of fresh M. bracteata or a mixture of C. citratus + M. bracteata extracts can be applied by spraying directly on the soil to inhibit weed emergence in the field. Further testing is needed through direct treatment of the extract on weeds at various growth stages to determine the effect of the extract on weed growth and mortality.
Acknowledgements
We express our gratitude for the support provided by the Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Riau, so that research can run smoothly.
Novelty Statement
Mixed extracts (fresh leaves of C. citratus + M. bracteata) were more effective than single extracts in inhibiting weed germination and growth.
Author’s Contribution
Siti Fatonah: planned the research, supervised the implementation of the research and wrote the publication.
Herman: assisted the research and proofread the writing for publication.
Dewi Yuni Safitri: conducted the research and compiled the research report.
Conflict of interest
The authors have declared no conflict of interest.
References
Abbas, T., Ahmad, A., Kamal, A., Nawaz, M.Y., Jamil, M.A., Saeed, T. and Ateeq, M., 2021. Ways to use allelopathic potential for weed management: A review. Int. J. Food Sci. Agric., 5: 492-498. https://doi.org/10.26855/ijfsa.2021.09.020
Abbas, T., Nadeem, M.A., Tanveer, A., Ali, H.H. and Farooq, N., 2018. Role of allelopathic crop mulches and reduced doses of tank-mixed herbicides in managing herbicide-resistant Phalaris minor in wheat. Crop Prot., 110: 245-250.https://doi.org/10.1016/j.cropro.2017.06.012
Abdullah, H., Abdullatif, Z. and Sudjud, S., 2020. Tomato and curly chili post-planting weed community composition. In 5th international conference on food, agriculture and natural resources (FANRes 2019). Atlantis Press.https://doi.org/10.2991/aer.k.200325.026
Abigail, J. and Dashak, D., 2020. Phytochemical screening and gas chromatography-mass spectroscopy analysis of methanolic extract of Mucuna bracteata seeds. Bima J. Sci. Technol., 4(2): 8-16.
Abouziena, H.F. and Haggag, W.M., 2016. Weed control in clean agriculture: A review. Planta Daninha., 34: 377-392. https://doi.org/10.1590/S0100-83582016340200019
Al-Harbi, N.A., 2018. Allelopathic effect of leaf extract of two wild plants on seed germination, shoot and root length of two weed species; Portulaca oleracea and Chenopodium murale. Biosci. Biotechnol. Res. Asia, 15(4): 929-935.https://doi.org/10.13005/bbra/2704
Bachheti, A., Sharma, A., Bachheti, R.K., Husen, A. and Pandey, D.P., 2020. Plant Allelochemicals and Their Various Applications. Co-evolution of secondary metabolites., pp. 441-465. https://doi.org/10.1007/978-3-319-96397-6_14
Bashar, H.M.K., Juraimi, A.S., Ahmad-Hamdani, M.S., Uddin, M.K., Asib, N., Anwar, M.P. and Hossain, A. 2022. Evaluating the phytotoxicity of methanolic extracts of Parthenium hysterophorus L. on selected crops and weeds. bioRxiv: 2022-01.
Bekuzarova, S.A., Khanieva, I.M., Lushchenko, G.V., Mamiev, D.M. and Tedeeva, A.A., 2020. Weeds biological control technique. In IOP Conference Series: Environ. Earth Sci., 548(8): 082008. https://doi.org/10.1088/1755-1315/548/8/082008
Caser, M., Demasi, S., Caldera, F., Dhakar, N.K., Trotta, F. and Scariot, V., 2020. Activity of Ailanthus altissima (Mill.) swingle extract as a potential bioherbicide for sustainable weed management in horticulture. Agronomy, 10(7): 965.https://doi.org/10.3390/agronomy10070965
Cheng, F. and Cheng, Z. 2015. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci., 6: 1020. https://doi.org/10.3389/fpls.2015.01020
Dahiya, S., Kumar, S., Khedwal, R.S. and Jakhar, S.R., 2017. Allelopathy for sustainable weed management. Res. J. Pharmacogn. Phytochem., SP1: 832-837.
Dahliani, L. and Elban, S., 2019. The dominant weed type in three areas of mature palm oil on peatland. In: Proceedings of the 1st international conference on engineering, science, and commerce, ICESC 2019, 18-19 October 2019, Labuan Bajo, Nusa Tenggara Timur, Indonesia. https://doi.org/10.4108/eai.18-10-2019.2289969
Farooq, N., Abbas, T., Tanveer, A. and Jabran, K., 2020. Allelopathy for weed management. In: Co-evolution of secondary metabolites: 505-519. Springer Nature Switzerland AG 2020.https://doi.org/10.1007/978-3-319-96397-6_16
Fatonah, S., Isda, M.N. and Herman. 2014. Potentials of fresh extract of Ageratum conyzoides L. and Piper betle L. as organic herbicide for Borreria alata (Aublet) DC. In: Prosiding Semirata 2014 of MIPA Field of West BKS-PTN. IPB Bogor, 9-11 May 2014.
Fatonah, S., Murtini, I. and Isda, M.N., 2013. Allelopathy potentials of leave extract of Pueraria javanica Benth. Toward germination and tiller growth of Asystasia gangetica (L.) T. Anderson. Prosiding of National Seminar of Biodiversity and Indonesian Tropical Ecology (BioETI) University of Andalas, Padang, 14 September 2013. pp. 21-27.
Fatonah, S. and Herman. 2011. Floristic composition of weeds in palm oil plantation with different age and control method at Tambang Village, Kampar. In: Prosiding Semirata 2011 of MIPA Field of West BKS-PTN. Unlam Banjarmasin, 9 – 10 May 2011.
Feng, C.H.E.N., Yongjie, M.E.N.G., Haiwei, S.H.U.A.I., Xiaofeng, L.U.O., Wenguan, Z. H.O.U., Jianwei, L.I.U. and Kai, S.H.U., 2017. Effect of plant allelochemicals on seed germination and its ecological significance. Chin. J. Eco-Agric., 25(1): 36-46.
Gbenou, J.D., Ahounou, J.F., Akakpo, H.B., Laleye, A., Yayi, E., Gbaguidi, F. and Kotchoni, S.O., 2013. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep., 40: 1127-1134. https://doi.org/10.1007/s11033-012-2155-1
Gupta, P.K., Rithu, B.S., Shruthi, A., Lokur, A.V. and Raksha, M., 2019. Phytochemical screening and qualitative analysis of Cymbopogon citratus. Res. J. Pharmacogn. Phytochem., 8(4): 3338-3343.
Hussain, M.I. and Reigosa, M.J., 2011. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot., 62(13): 4533-4545. https://doi.org/10.1093/jxb/err161
Ishak, M.S. and Sahid, I., 2014. Allelopathic effects of the aqueous extract of the leaf and seed of Leucaena leucocephala on three selected weed species. In: AIP conference proceedings. Am. Inst. Phys., 1614(1): 659-664. https://doi.org/10.1063/1.4895280
Ismail, B.S.I., Halimshah, S.W.A. and Yusoff, N., 2016. Allelopathic potential of the leaf and seed of Pueraria javanica Benth. on the germination and growth of three selected weed species. Sains Malays., 45(4): 517-521.
Jha, P., Kumar, V., Godara, R.K. and Chauhan, B.S., 2017. Weed management using crop competition in the United States: A review. Crop Prot., 95: 31-37.https://doi.org/10.1016/j.cropro.2016.06.021
Khalaj M.A., Amiri M. and Azimi, M.H., 2013. Allelopathy: Physiological and Sustainable Agriculture Important Aspects. Int. J. Agron. Plant Prod., 4(5): 950-962.
Khamare, Y., Chen, J. and Marble, S.C., 2022. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci., 13: 1034649.https://doi.org/10.3389/fpls.2022.1034649
Khan, A.U., Ullah, F., Mehmood, S., Irshad, M. and Khan, F.U., 2017. Allelopathic effects of Jatropha curcas L. leaf aqueous extract on early seedling growth of Parthenium hysterophorus L. Pak. J. Agric. Sci., 30(1): 45-54.
Kostina-Bednarz, M., Płonka, J. and Barchanska, H., 2023. Allelopathy as a source of bioherbicides: challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol., pp. 1-34. https://doi.org/10.1007/s11157-023-09656-1
Kubiak, A., Wolna-Maruwka, A., Niewiadomska, A. and Pilarska, A.A., 2022. The problem of weed infestation of agricultural plantations vs. the assumptions of the European biodiversity strategy. Agronomy, 12(8): 1808. https://doi.org/10.3390/agronomy12081808
Kumar, A., Rajput, G., Dhatwalia, V. K., and Srivastav, G., 2009. Phytocontent screening of Mucuna seeds and exploit in opposition to pathogenic microbes. J. Biol. Environ. Sci., 3(9): 71-76.
Kurniadie, D. and Umiyati, U., 2019. Effect of rainfastness of herbicide potassium glyphosate to control weeds of oil palm. Res. Crops, 20(4): 719-724. https://doi.org/10.31830/2348-7542.2019.106
Latif, S., Chiapusio, G. and Weston, L.A., 2017. Allelopathy and the role of allelochemicals in plant defence. Adv. Bot. Res., 82: 19-54. https://doi.org/10.1016/bs.abr.2016.12.001
Li, Z., Wang, Q., Ruan, X., Pan, C. and Jiang, D., 2010. Review, phenolics and plant allelopathy. Molecules., 15: 8933-8952. https://doi.org/10.3390/molecules15128933
Li, J., He, Y., Ma, D., He, B., Wang, Y. and Chen, B., 2018. Volatile allelochemicals of Chenopodium ambrosioides L. induced mitochondrion-mediated Ca 2+-dependent and Caspase-dependent apoptosis signaling pathways in receptor plant cells. Plant Soil, 425: 297-308.https://doi.org/10.1007/s11104-018-3593-x
M’barek, K., Zribi, I., Ullah, M.J. and Haouala, R., 2019. The mode of action of allelochemicals aqueous leaf extracts of some Cupressaceae species on lettuce. Sci. Hortic., 252: 29-37.https://doi.org/10.1016/j.scienta.2019.03.009
Marin-Morales, M.A., Ventura-Camargo, B.D.C. and Hoshina, M.M., 2013. Toxicity of herbicides: Impact on aquatic and soil biota and human health. Herbicides current research and case studies in use. 10: 55851.
Monteiro, A. and Santos, S., 2022. Sustainable approach to weed management: The role of precision weed management. Agronomy, 12(1): 118. https://doi.org/10.3390/agronomy12010118
Mushtaq, M.N., Cheema, Z.A. and Khaliq, A., 2010. Effects of mixture of allelopathic plant aqueous extracts on Trianthema portulacastrum L. weed. Allelopathy J., 25(1): 205-212.
Natarajan, K., Narayanan, N. and Ravichandran, N., 2012. Review on Mucuna. The wonder plant. Int. J. Pharm. Sci. Rev. Res., 17(1): 86-93.
Ni, L., Rong, S., Gu, G., Hu, L., Wang, P., Li, D. and Li, S., 2018. Inhibitory effect and mechanism of linoleic acid sustained-release microspheres on Microcystis aeruginosa at different growth phases. Chemosphere, 212: 654-661.https://doi.org/10.1016/j.chemosphere.2018.08.045
Novakoski, A.D.S., Coelho, É.M.P., Ravagnani, G.T., Costa, A.C.P.R.D., Rocha, S.A., Zucareli, V. and Lopes, A.D., 2020. Allelopathic potential of plant aqueous mixtures on Euphorbia heterophylla. Agriculture, 10(10): 1-14. https://doi.org/10.3390/agriculture10100449
Ogunsusi, M., Akinlalu, A.O., Komolafe, I.J. and Oyedapo, O.O., 2018. Allelopathic effects of alkaloid fraction of Crotalaria retusa Linn on growth and some biochemical parameters of bean seedlings Phaseolus vulgaris. Int. J. Plant Physiol. Biochem., 10(1): 1-9. https://doi.org/10.5897/IJPPB2017.0261
Oladeji, O.S., Adelowo, F.E., Ayodele, D.T. and Odelade, K.A., 2019. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr., 6: e00137. https://doi.org/10.1016/j.sciaf.2019.e00137
Omezzine, F., Ladhari, A. and Haouala, R., 2014. Physiological and biochemical mechanisms of allelochemicals in aqueous extracts of diploid and mixoploid Trigonella foenum-graecum L. S. Afr. J. Bot., 93: 167-178. https://doi.org/10.1016/j.sajb.2014.04.009
Oracz, K., Bailly, C., Gniazdowska, A., Côme, D., Corbineau, F. and Bogatek, R., 2007. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J. Chem. Ecol., 33: 251-264. https://doi.org/10.1007/s10886-006-9222-9
Pagare, S., Bhatia, M., Tripathi, N., Pagare, S. and Bansal, Y.K., 2015. Secondary metabolites of plants and their role: Overview. Curr. Trends. Biotechnol. Pharm., 9(3): 293-304.
Patil, H.S., Shitole, S.M. and Dhumal, K.N., 2013. Effect of leaf and root extracts of selected weed species on seed germination and seedling growth in mung bean. Int. J. Curr. Res., 5(1): 94-98.
Petit, S., Boursault, A., Le Guilloux, M., Munier-Jolain, N. and Reboud, X., 2011. Weeds in agricultural landscapes. A review. Agron. Sustain. Dev., 31: 309-317. https://doi.org/10.1051/agro/2010020
Quarles, W., 2010. Alternative herbicides in turfgrass and organic agriculture. IPM Practitioner, XXXII(5/6).
Ramachandran, A. and Venkataraman, N.S., 2016. Allelopathic effects of aqueous leaf extracts of Datura metel L. on Parthenium hysterophorus L. Life Sci. Leaflets. 72: 14-22.
Rane, M., Suryawanshi, S., Patil, R., Aware, C., Jadhav, R., Gaikwad, S. and Jadhav, J., 2019. Exploring the proximate composition, antioxidant, anti-Parkinson's and anti-inflammatory potential of two neglected and underutilized Mucuna species from India. S. Afr. J. Bot., 124: 304-310. https://doi.org/10.1016/j.sajb.2019.04.030
Rao, A.N. and Matsumoto, H., 2017. Weed management in rice in the Asian-Pacific region. Asian-pacific weed science society (APWSS). The Weed Science Society of Japan, Japan and Indian Society of Weed Science, India.
Rojas-Sandoval, J., 2018. Paspalum conjugatum (buffalo grass). Crop Protection Compendium: 38951
Scavo, A. and Mauromicale, G., 2021. Crop allelopathy for sustainable weed management in agroecosystems: Knowing the present with a view to the future. Agronomy, 11(11): 2104. https://doi.org/10.3390/agronomy11112104
Sharma, D.D., Chinnaswamy, C. and Singh, S.M., 2020. Weed management in horticulture crops. Agrimoon.com.
Sharma, N., and Rayamajhi, M. 2022. Different aspects of weed management in maize (Zea mays L.): A brief review. Advances in Agriculture: 2022.https://doi.org/10.1155/2022/7960175
Sihombing, A., Fatonah, S. and Silviana, F., 2012. Allelopathic effect of Calopogonium mucunoides Desv. on germination and seedling growth of Asystasia gangetica (L.) T. Biospecies, 5(2): 5-11.
Sisodia, S. and Siddiqui, M.B., 2010. Allelopathic effect by aqueous extracts of different parts of Croton bonplandianum Baill. on some crop and weed plants. J. Agric. Ext. Rural Dev., 2(1): 22-28.
Skinner, E.M., Díaz-Pérez, J.C., Phatak, S.C., Schomberg, H.H. and Vencill, W., 2012. Allelopathic effects of sunnhemp (Crotalaria juncea L.) on germination of vegetables and weeds. HortSci., 47(1): 138-142. https://doi.org/10.21273/HORTSCI.47.1.138
Smith-Fiola, D. and Gill, S., 2014. Vinegar: An alternative to glyphosate? Central maryland research and education center. The University of Maryland, College of Agriculture and Natural Resources.
Sofiah, S., 2008. Cymbopogon citratus as one of essential oil producers. UPT of Plant Conservation Office. Purwodadi Botanical Garden of LIPI. http:// wordpress.com/budidaya-serai-dapur.
Soltys, D., Krasuska, U., Bogatek, R. and Gniazdowska, A., 2013. Allelochemicals as bioherbicides. Present and perspectives. In Herbicides-Current research and case studies in use. IntechOpen. https://doi.org/10.5772/56185
Staszek, P., Krasuska, U., Ciacka, K. and Gniazdowska, A., 2021. ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants, 10(11): 1648.https://doi.org/10.3390/antiox10111648
Suwal, M.M., Devkota, A. and Lekhak, H.D., 2010. Allelopathic effects of Chromolaena odorata (L.) king and robinson on seed germination and seedlings growth of paddy and barnyard grass. Sci. World J., 8(8): 73-75. https://doi.org/10.3126/sw.v8i8.3854
Tanase, C., Bujor, O.C. and Popa, V.I., 2019. Phenolic natural compounds and their influence on physiological processes in plants. In: Polyphenols in plants. Academic Press.https://doi.org/10.1016/B978-0-12-813768-0.00003-7
Taupik, S.A.M., Aani, S.N.A., Wai, C.P. and Seng, C.T., 2022. Allelopathic potential of cassava (Manihot esculenta L.) extracts on germination and seedling growth of selected weeds and aerobic rice. Sains Malays., 51(3): 633-642.https://doi.org/10.17576/jsm-2022-5103-01
Travlos, I., Gazoulis, I., Kanatas, P., Tsekoura, A., Zannopoulos, S., and Papastylianou, P., 2020. Key factors affecting weed seeds' germination, weed emergence, and their possible role for the efficacy of false seedbed technique as weed management practice. Front. Agron., 2: 1.https://doi.org/10.3389/fagro.2020.00001
Woranoot, K., Naree, P., Kongbangkerd, A., Wongkrajang, K., Buaruaeng, R. and Choopayak, C., 2015. Phytotoxic effects of Piper betle L. extracts on germination of Eclipta prostrata L. and Chloris barbata Sw. weeds. NU. 12(1): 11-24.
Zeng, R. S., Luo, S. M., Shi, Y. H., Shi, M. B., and Tu, C. Y., 2001. Physiological and biochemical mechanism of allelopathy of secalonic acid F on higher plants. Agronomy journal, 93(1), 72-79. https://doi.org/10.2134/agronj2001.93172x
Zhang, S., Sun, S.W., Shi, H.L., Zhao, K., Wang, J., Liu, Y. and Wang, W., 2020. Physiological and biochemical mechanisms mediated by allelochemical isoliquiritigenin on the growth of lettuce seedlings. Plants, 9(2): 245. https://doi.org/10.3390/plants9020245
To share on other social networks, click on any share button. What are these?







