A Comparison of Amino Acid Digestion of Crossbred Ducks (Super Meat x Pekin Ducks) Supplemented Shrimp By-Products
Research Article
A Comparison of Amino Acid Digestion of Crossbred Ducks (Super Meat x Pekin Ducks) Supplemented Shrimp By-Products
Nguyen Thi Kim Dong1*, Nguyen Van Thu2 , Nguyen Hoang Qui3
1Tay Do University, No. 68 Tran Chien Street, Le Binh ward, Cai Rang district, Can Tho city, Vietnam; 2Can Tho University, 3/2 street, NinhKieu district, Can Tho city, Vietnam; 3TraVinh University, No. 126 Nguyen ThienThanh Street, Ward 4, District 5, TraVinh City, TraVinh Province, Vietnam.
Abstract | Ninety-six crossbred ducks fed with shrimp by-products (SBP) were used in this experiment, in which four experimental diets were fed following a factorial design, with 3 replicates of 4 ducks (1:1 sex ratio) in one experimental unit. The first factor was the digestion technique (ileal and total tract digestibility) and the second factor was the diets. Four diets included a basal diet (SBP0), which consisted of fish meal 20% (FM), broken rice 80% (BR) and the other three diets, in which the content of FM was 25.0, 50.0 and 100% of the FM level in the basal diet and was replaced by ensiled shrimp by-products corresponding the SBP1, SBP2 and SBP3 diet, respectively. Daily intakes of ether extract (EE) and fiber fractions increased with increasing levels of SBP. The apparent total tract digestibility of dry matter (DM), organic matter (OM), ether extract (EE), and nitrogen-free extract (NFE), as well as nitrogen retention, decreased linearly with increasing SBP (P <0.05). The apparent total tract digestibility of the majority of individual amino acids was greater than their apparent ileal digestibility (P <0.05). Individual amino acid digestibility decreased linearly (P<0.05) as the dietary level of SBP increased
Keywords | Crossbred duck, amino acids, ileal and excreta digestibility, shrimp by-products,
Received | January 21, 2023; Accepted | February 20, 2023; Published | March 07, 2023
*Correspondence | Nguyen Thi Kim Dong, Tay Do University, No. 68 Tran Chien Street, Le Binh ward, Cai Rang district, Can Tho city, Vietnam; Email: ntkdong@ctu.edu.vn
Citation | Dong NTK, Thu NV, Qui NH (2023) .A comparison of amino acid digestion of crossbred ducks (super meat x pekin ducks) supplemented shrimp by-products adv. Anim. Vet. Sci. 11(4): 558-567.
DOI | http://dx.doi.org/10.17582/journal.aavs/2023/11.4.558.567
ISSN (Online) | 2307-8316

Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
In recent years duck flocks in Vietnam were boosted for both quality and quantity because of its economic values for small farmers. Linh et al. (2022) showed that local ducks were widely raised in Mekong delta and have improved income for producers. The most pressing concern is to enhance the production of duck production while maintaining current manufacturing costs. Utilizing agricultural waste products in this manner would be possible. Shrimp head meal or shrimp by-products can be utilized as an additional source of protein for poultry in place of fish meal (Pagala et al., 2021). In Vietnam one of the most significant exports is shrimp production with the productivity of 980,000 tons in 2022, in which shrimp by-products accounted about 50%. This waste makes up a significant portion of the weight of all raw shrimp and is a valuable resource that may be recycled into new commodity with increased value through animal production. The shells and exoskeletons of shrimp are referred to as industrial waste from shrimp-freezing factories and can account for 45–48% of the intact weight of the shrimp, with adequate protein and phosphorus content (Mao et al., 2017; Kumari and Rupak, 2020). It is necessary to have access to accurate estimates of nutrient digestibility in order to use shrimp waste as an animal feed resource as efficiently as possible, due to its ease and the possibility of using a large number of birds without having to sacrifice any of them, excreta analysis is the basis for the bulk of published figures on digestible amino acids for poultry at this time (McNab, 1994). Hydrolysis and fermentation were two further techniques for processing shrimp waste. From fishing wastes, biological and chemical silages are created and effectively employed in animal feeding. Ngoan et al. (2000) demonstrated that shrimp meal’s amino acid composition was generally balanced. Shrimp meal must be treated to enhance its nutritional value in poultry diets since broiler digestive tracts do not produce enough chitinase (Rezvani et al., 2017). The concentrations of the head and shell may have an impact on how helpful shrimp excrement is in diets (Brito et al., 2020). Additionally, shrimp waste meal had 30.4% crude protein, 61.2% organic matter, and 38.8% ash (Brito et al., 2020), while fish meal contained 60.2% CP, 79.5% OM and 20.5% ash (Linh et al., 2022). Shrimp waste can be added to feed as a supplement to cut waste and production costs. While adding shrimp waste meal to the diet of free-range hens can increase digestibility, using excessive amounts of shrimp waste meal in growing broiler chicken reduced DM digestibility and ME values. According to the study by Khempaka et al. (2006), shrimp meal can be used as a source of protein in a broiler diet if it is included at and below 4%.
Although the apparent nutrient digestibility of shrimp meal has been reported (Khempaka et al., 2006), there has been little research on ensilaged shrimp by products; therefore, the present study also aimed to determine the effects of supplementing ensilaged shrimp by-products on nutrient and amino acid digestibility of crossbred ducks in Vietnam.
Materials and Methods
Location and ethical approval
The experiment was conducted at the experimental farm of Can Tho University, Vietnam. It included ninety-six 12-weeks-old crossbred ducks (male Super Meat crossbred with female Pekin ducks) with an average live weight of 1.8 kg to 1.9kg. All procedures were approved by CanTho University and followed the Vietnam’s standard regulation for animal’ cares.
Experimental design
A total of 96 crossbred ducks were assigned in a factorial design with 3 replicates of 4 ducks in one experimental unit to determine the ileal and total tract amino acid digestion. The first factor was the digestion technique (ileal and total tract amino acid digestibility) and the second factor was the diets. Four diets included a basal diet (SBP0) which consisted of fish meal 20% (FM), broken rice 80% (BR) and the other three diets in which the content of FM was 25.0, 50.0 and 100% of the FM level in the basal diet and was replaced by ensiled shrimp by-products corresponding the SBP1, SBP2 and SBP3 diet, respectively. This study was a factorial design, however, the nutrient digestibility was measured only for the total tract (excreta). Because the ileal digesta amount per experimental unit was small, being not enough for measurement of nutrient digestibility. The ducks were randomly assigned to one of 24 wire metabolism cages (0.5 x 1.0 x 0.8 meters), each containing two males and two females of the same mean weight in each cage. A plastic tray was placed underneath each cage to collect any and all feces that may have been produced. A drinker and a feeder were also provided in each cage. The cages were put in a well-ventilated room that was illuminated continuously to facilitate eating throughout the night. Every morning began with a thorough cleaning of the cages, feeders, drinkers, and plastic trays.
Diets and feeding
Fresh shrimp by-products were collected on one occasion from a local marine product processing factory in Can Tho City, ground and ensiled by mixing with molasses at a ratio of 3:1 (3 units of shrimpby-products to 1 of molasses on a wet weight basis). The mixture was then placed in plastic bags and put into plastic buckets that were carefully sealed to prevent air contamination. After 2 weeks the ensiled shrimp by-products was dried, ground and stored for later use.
Tables 1 and 2 show, respectively, the components of the experimental diets in terms of their chemical composition as well as the ingredients. The basal diet, SBP0, consisted of 80% bran rice (BR) and 20% fish meal (FM). Both the broken rice and the fish meal that was included in the diets that were being tested came from the same batch. For the other three diets, 25, 50, and 100% of the fish meal in the basal diet was replaced by shrimp by-products, giving diets SBP1, SBP2, and SBP3, respectively (Table 2). A vitamin-mineral premix (0.3%) was added to all diets and chromic oxide (0.3%) was used as an indigestible marker (Kim et al., 2012; Foltyn et al., 2015, Han et al., 2017). The premix and chromic oxide were mixed carefully with the other feed ingredients immediately before feeding.
The diets were introduced gradually during seven days, followed by a period of adaptation lasting seven days, and then a period of experimentation lasting five days. In order to reduce the amount of SBP spillage, the ducks were fed in groups of four at four different times throughout the day (at 8:00, 13:00, 17:00, and 21:00 h). During the adaptation period of seven days, the ducks were allowed to eat adlibitumin order to accurately measure its feed intake. In order to reduce feed refusals during the collection period, the feeding level was set slightly below that of the adaptation period. The ducks’ access to water was restricted, allowing them to consume any feed remaining in the drinkers.
Sampling procedure for excreta and ileal digesta
During the 5-day collection period, feed samples and total excreta were collected for the purpose of calculating actual feed and nutrient consumption and conducting chemical analysis. Excreta were collected quantitatively three times daily at 7:00 am, 13:00 pm, and 18:00 pm, then frozen at –20 degrees Celsius. To prevent contamination with scales, feathers, etc. precautions were taken. Excreta were frozen, pooled within each diet and replicate, and dried at 60 degrees Celsius for 24 hours prior to analysis. Excreta were weighed, ground and homogenized to pass a 0.50 mm sieve, and representative samples were collected and stored at -4 degrees Celsius in airtight plastic containers (Ravindran et al., 1999).
At the end of the collection period, four hours following the last feeding, birds were killed by cervical dislocation in the same sequence in which they had been fed. It has been determined that a 4-hour delay is best for ileal digesta sample in broiler chickens. The ileum was dissected out within 5 minutes after slaughter and is defined as extending from Meckel’s diverticulum to the ileo-cecal junction. Ileal digesta was quantitatively collected by careful flushing, and then placed in plastic bags that were sealed. The ileal contents of four ducks in a pen were combined to gather three samples per feeding treatment. The collected digesta were promptly frozen and then dehydrated. Drying and grinding ileal digesta samples followed the same technique as for excreta samples. It was allowed to oven-dry digesta and excreta samples at a low temperature (55-600C) for 24 hours. The ileal contents of four ducks in a pen were immediately frozen after collection and then dried. The procedure of drying and grinding of the ileal digesta samples was similar to that for excreta samples. Oven-drying digesta and excreta samples at low temperature (55-600C) for 24 hours was considered to be acceptable.
Chemical analyses
Standard AOAC procedures were used to evaluate BR, FM, SBP, and diet samples for DM, N, and CP (N*6.25), EE, CF, ash, calcium, and phosphorus (AOAC, 1990). Excreta and ileal digesta were also tested for DM, EE, and N using the procedures described above. NDF and ADFwere analyzed using the method of Goering and Van Soest (1991). The chitin content of the ensiled shrimp by-products was determined using an enzymatic approach including pure chitinase (Jeuniaux and Voss-Foucart, 1997). The amino acid content of representative samples of BR, FM, and shrimp by-products, mixed meals, excreta, and ilealdigesta were evaluated (AOAC, 2000). Diets, excreta, and ilealdigesta were examined for Cr2O3content (Takemasa, 1992) in order to calculate amino acid digestibility.
Measurements taken
Individual bird weights were taken at the beginning of the study. The feed intake was daily determined by the total amount of food consumed by four ducks per cage. Excreta and ileal digesta were quantitatively collected in groups.
Excreta and apparent ileal amino acid digestibility (AAAD) were computed using Cr2O3 as indigestible marker (Ravindran et al., 2002; Bryden et al., 2009) as shown below:
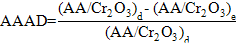
Where (AA/ Cr2O3)d presentsthe ratio of amino acid to indigestible marker in the diet
(AA/ Cr2O3)ie represents the ratio of amino acid to indigestible marker in the ilealdigesta (or excreta).
Apparent N retention was calculated as the difference between N intake and N in excreta (including uric acid N).
Data analysis
The data were evaluated by analysis of variance using the Minitab Reference Manual Release 16.1.0, ANOVA General Linear Model technique (2010), when the F-test was significant at P<0.05, Tukey’sprocedure was used to conduct paired comparisons.
Digestible AA data (AAdig were analyzed by the GLM procedure (SAS application) using the following model:
(1) Proc GLM; Class Method; Model AAdig = Method SBP (Method) / SS3 solution;
Where Method is ileal or excreta digestibility and SBP is the dietary content of SBP (%).
The interaction of the ileal and excreta regression on SBP was tested using the model:
(2) Proc GLM; Class Method; Model AAdig = Method SBP Method * SBP/SS3;
Where no significant interactions were found according to model (2) model (3) was used for extrapolation to 100 % of SBP.
(3) Proc GLM; Class Method; Model AAdig= Method SBP/SS3 solution;
Where interactions were present equations from model (1) were used.
Results
Chemical composition and nutrient intakes
The analysed chemical composition of feed ingredients is presented in Table 2 and the calculated composition of the experimental diets is shown in Table 3. The results indicat
Table 1: Ingredient composition containing in the experimental diets (%DM)
|
Ingredient |
Diet1 |
|||
| SBP3 |
SBP2 |
SBP1 |
SBP0 |
|
| Broken rice (BR) | 69.6 | 74.4 | 77.1 | 80.0 |
| Fish meal (FM) | 0 | 9.30 | 14.5 | 20.0 |
| Shrimp by-products (SBP) | 30.4 | 16.3 | 8.40 | 0 |
|
Vit-mineral premix2 |
0.30 | |||
1 SBP0, SBP1, SBP2, SBP3: 0, 25, 50 and 100% of FM replaced by SBP.
Vit: Vitamin
2 Vit-mineral premix includesvitD3: 80,000 IU/100g; vit A: 50,000 IU/100g; vitE:50 IU/100 g; vit B1: 0.03%; vit K: 0.1%; vit B2: 0.2%; vit B12: 0.0006%; calcium carbonate: 0.5%; Cu: 0.32%; Phosphorus: 0.05%; Zn: 1.6%; Mn: 2.56%; Se: 0.0064%; I: 0.032%; Co: 0.016%.
Table 2: Analyzed feed ingredient’s chemical composition (%DM)
| Criteria |
BR |
FM |
Ensiled SBP |
| DM | 88.4 | 85.4 | 89.9 |
| OM | 99.6 | 75.7 | 82.6 |
| CP | 11.9 | 57.9 | 31.2 |
| EE | 1.12 | 11.4 | 12.6 |
| NFE | 86.5 | 5.52 | 31.8 |
| CF | 0.12 | 0.85 | 7.04 |
| NDF | 6.37 | 1.79 | 6.86 |
| ADF | 1.97 | 1.07 | 5.86 |
|
Ash |
0.35 | 24.3 | 17.4 |
| Chitin | - | - | 13.2 |
| Ca | 0.16 | 4.60 | 5.37 |
| P | 0.20 | 1.91 | 1.46 |
| ME, MJ/kg | 14.4 | 12.5 |
10.3 |
DM: dry matter, OM: organic matter, CP: crude protein, EE: ether extract, NFE: nitrogen free extract, CF: crude fibre, NDF: neutral detergent fiber, ADF: acid detergent fiber and ME: Metabolizable energy
Table 3: Calculated chemical composition of the experimental diets (%, DM basis)
|
Item |
Diet1 |
|||
| SBP3 |
SBP2 |
SBP1 |
SBP0 |
|
| DM | 88.8 | 88.3 | 88.0 | 87.8 |
| OM | 94.5 | 94.6 | 94.7 | 94.9 |
| CP | 17.8 | 19.3 | 20.2 | 21.1 |
| EE | 4.65 | 3.99 | 3.61 | 3.22 |
| NFE | 69.8 | 70.0 | 70.1 | 70.3 |
| CF | 2.22 | 1.31 | 0.80 | 0.26 |
| NDF | 6.52 | 6.02 | 5.74 | 5.45 |
| ADF | 3.15 | 2.52 | 2.16 | 1.79 |
| Ash | 5.53 | 5.36 | 5.26 | 5.15 |
| ME, ME/ kg DM | 13.2 | 13.6 | 13.8 | 14.0 |
1 Compared with Table 1 and Table 2.
ed that the CP and ME contents of SBP were considerably lower than those in the FM, whereas the content of fibrous components was higher in SBP. The chitin content of SBP was 13.2% on DM basis. This resulted in the lowest CP and the highest fibre contents in the SBP3 diet.
Table 4: Content of amino acids: Analyzed in the feed ingredients and calculated in the experimental diets (% of DM) and recommended Ideal Protein composition.
|
Item |
Ideal Protein2 |
Ingredients |
Diets |
||||||
SBP |
SBP1 |
FM |
BR |
SBP3 |
SBP2 |
SBP1 |
SBP0 |
||
| Essential amino acids | |||||||||
| Lysine | 100 | 1.67 | 100 | 4.47 | 0.33 | 0.74 | 0.94 | 1.05 | 1.16 |
| Arginine | 100 | 1.54 | 92 | 3.60 | 0.85 | 1.06 | 1.22 | 1.30 | 1.40 |
| Isoleucine | 77 | 1.77 | 106 | 3.01 | 0.51 | 0.89 | 0.94 | 0.97 | 1.01 |
| Leucine | 130 | 2.15 | 128 | 3.36 | 0.80 | 1.21 | 1.26 | 1.29 | 1.31 |
|
Methionine + cystine |
75 | 0.81 | 48 | 1.99 | 0.39 | 0.52 | 0.61 | 0.66 | 0.71 |
| Histidine | 43 | 1.17 | 70 | 1.66 | 0.31 | 0.57 | 0.57 | 0.58 | 0.58 |
| Phenylalanine | 120 (Phe+Tyr) | 1.00 | 60 | 2.38 | 0.59 | 0.72 | 0.82 | 0.89 | 0.95 |
| Threonine | 66 | 1.25 | 75 | 3.10 | 0.53 | 0.75 | 0.88 | 0.96 | 1.04 |
| Tyrosine | - | 1.28 | 76 | 1.58 | 0.57 | 0.78 | 0.78 | 0.77 | 0.77 |
| Valine | 89 | 1.57 | 94 | 2.64 | 0.51 | 0.83 | 0.88 | 0.91 | 0.94 |
| Total EAA | - | 14.2 | 27.8 | 5.39 | 8.07 | 8.90 | 9.38 | 9.87 | |
| Non-essential amino acids | |||||||||
| Alanine | - | 5.62 | 336 | 3.76 | 0.65 | 2.16 | 1.75 | 1.52 | 1.28 |
|
Aspartic acid |
- | 3.55 | 212 | 4.30 | 0.47 | 1.40 | 1.32 | 1.28 | 1.23 |
| Glutamic acid | - | 1.63 | 97 | 5.67 | 0.96 | 1.16 | 1.51 | 1.70 | 1.90 |
| Glycine | 127 | 2.67 | 159 | 2.03 | 0.52 | 1.17 | 1.01 | 0.92 | 0.82 |
| Proline | - | 1.81 | 108 | 4.00 | 0.38 | 0.81 | 0.95 | 1.02 | 1.10 |
| Serine | - | 1.05 | 63 | 2.39 | 0.51 | 0.68 | 0.77 | 0.83 | 0.89 |
| Sum of amino acids (SAA) | - | 30.5 | 49.9 | 8.9 | 15.5 | 16.2 | 16.7 | 17.1 | |
| EAA : SAA | - | 0.47 | 0.56 | 0.61 | 0.52 | 0.55 | 0.56 |
0.58 |
|
* Values were calculated based on amino acids of feed ingredients
1 SBP analyzed values (lysine as 100), 2 Ideal Protein for growing ducks, Rose (1997).
Table 5: Dry matter and nutrient intakes of ducks fed diets containing different levels of shrimp by-products (SBP) in excreta digestion technique.
| Item |
Diet1 |
SEM/P |
|||
| SBP3 |
SBP2 |
SBP1 |
SBP0 |
||
| Daily intake, g/d | |||||
| DM | 93.1 | 87.2 | 85.4 | 83.7 | 5.94/ 0.70 |
| OM | 87.9 | 82.6 | 80.9 | 79.4 | 5.62/ 0.73 |
| CP | 16.5 | 16.8 | 17.2 | 17.6 | 1.14/ 0.90 |
| EE |
4.33a |
3.48b |
3.08ab |
2.69c |
0.24/ 0.007 |
| NFE | 65.0 | 61.1 | 59.9 | 58.8 | 4.16/ 0.74 |
| CF |
2.07a |
1.15b |
0.69c |
0.22d |
0.09/ 0.001 |
| NDF | 6.07 | 5.26 | 4.91 | 4.56 | 0.36/ 0.08 |
| ADF |
2.93a |
2.19b |
1.85ab |
1.49c |
0.16/ 0.001 |
| ME, MJ/ d | 1.23 | 1.19 | 1.18 | 1.17 |
0.08/ 0.96 |
1 See Table 1
a, b, cDifferent superscripts in the same row showed a significant difference (p<0.05)
Table 6: Dry matter and nutrient intakes of ducks fed diets containing different levels of the shrimp by-products (SBP) in ileal digestion technique
| Item |
Diet1 |
SEM / P |
|||
| SBP3 |
SBP2 |
SBP1 |
SBP0 |
||
| Daily intake, g/d | |||||
| DM | 94.2 | 88.3 | 86.4 | 84.7 | 4.89/ 0.81 |
| OM | 88.3 | 83.0 | 81.3 | 79.8 | 6.03/ 0.68 |
| CP | 16.7 | 17.0 | 17.4 | 17.8 | 1.22/ 0.10 |
| EE |
4.37a |
3.51b |
3.11ab |
2.72c |
0.33/ 0.007 |
| NFE | 65.8 | 61.8 | 60.6 | 59.5 | 3.95/ 0.80 |
| CF |
2.09a |
1.16b |
0.70c |
0.22d |
0.08/ 0.001 |
| NDF | 6.12 | 5.31 | 4.95 | 4.60 | 0.41/ 0.08 |
| ADF |
2.95a |
2.21b |
1.87ab |
1.50c |
0.19/ 0.001 |
| ME, MJ/ d | 1.23 | 1.20 | 1.19 | 1.18 |
0.07/ 0.92 |
1 See Table 1
a, b, c Different superscripts in the same row showed a significant difference (p<0.05)
Table 7: Apparent total tract digestibility of nutrients and nitrogen retention in ducks fed meals containing SBP in the excreta digestion technique
|
Item |
Diets |
SEM/ P |
|||
| SBP3 |
SBP2 |
SBP1 |
SBP0 |
||
| DM |
92.3b |
94.2ab |
95.9 a |
97.4a |
0.76/0.008 |
| OM |
93.0b |
94.9ab |
96.7a |
97.7a |
0.64/0.004 |
| EE |
76.4b |
83.9a |
86.4a |
89.5a |
1.70/0.003 |
| NFE |
42.9b |
53.0ab |
69.2ab |
78.5a |
7.74/0.046 |
| N intake, g/ d | 2.65 | 2.69 | 2.76 | 2.82 | 0.18/0.91 |
| N retention, g/d | 1.86 | 1.89 | 2.19 | 2.32 | 0.13/0.10 |
| N ret/ N int, % | 70.0 | 70.3 | 80.3 | 82.3 |
0.035/0.07 |
a, b Different superscripts in the same row showed a significant difference (p<0.05)
N ret: N retention, N int: N intake
According to the analysed amino acid composition of feed ingredients and the calculated amino acid composition of the experimental diets (Table 4), total essential amino acid (EAA) and non-essential amino acid (NEAA) concentrations were higher in FM than in SBP, which was reflected in the corresponding diets. The majority of EAA concentrations in SBP protein exceed the optimal protein composition for growing ducks, with the exception of sulphur AA, which are around 25% units below the recommendation.
In Table 5 and 6 the ducks for both ileal and excreta digestion techniques had similar tendency of the results and showed that the daily feed intakes were almost resemble among the treatments (p>0.05). The intakes of individual nutrients varied according to diet composition and DM intake. The highest intake of EE, CF and ADF was found on the SBP3 diet (p< 0.01), as a result of high contents of these components in the SBP.
Nutrients’ apparent total tract digestibility and nitrogen retention
Table 7 presents the apparent coefficients of total tract digestibility (ACTTD) and nitrogen retention. The ACTTD of DM, OM, EE, and NFE were greatest on the baseline diet (SBP0), then declined linearly with increasing amounts of SBP in the diets, with the lowest value on the SBP3 diet (p<0.01). The degree of depression was higher for EE and NFE than for other nutrients in the diet.
The digestibility of nutrients in SBP was estimated from regression equations (Table 8), and the estimated digestibility values of all nutrients in SBP were considerably lower compared to the experimental diets. The negative NFE digestibility values in SBP were probably a result of its high chitin content. There were no treatment effects on N intake (N-int) (p>0.05), but N retention (N-ret) and the ratio of N retention to N intake decreased linearly with increasing SBP levels in the diets as illustrated by the regression analyses. This also may be related to intestinal physiochemical
Table 8: Apparent total tract digestibility of nutrients and N retention in SBPand the regression equation of SBP on digestibility and N retention.
| Item |
Intercept |
SD* |
Slope |
P |
R2 |
SBP3** |
|||||||
| Total tract digestibility, % | |||||||||||||
| DM | 97.2 | 1.20 | -0.158 | 0.001 | 0.74 | 81.3 | |||||||
| OM | 97.7 | 1.02 | -0.150 | 0.001 | 0.78 | 82.7 | |||||||
| EE | 89.8 | 2.67 | -0.407 | 0.001 | 0.79 | 49.1 | |||||||
| NFE | 76.8 | 12.5 | -1.134 | 0.01 | 0.57 | -36.7 | |||||||
| N-ret, g/d | 2.27 | 0.23 | -0.015 | 0.022 | 0.42 | 0.76 | |||||||
| N-ret/N-int, % | 81.4 | 6.32 | -0.405 | 0.025 | 0.40 | 40.9 | |||||||
** SBP3: extrapolation to 100% SBP
* SD: standard error
Table 9: Apparent ileal and excreta digestibility of amino acids of ducks fed diets containing different levels of SBP
| Item |
Method (M) |
Diet1 |
SEM / P |
||||||
| Ileal * |
Exc. * |
SBP3 |
SBP2 |
SBP1 |
SBP0 |
Method |
Diet |
M*D |
|
| Essential amino acid (EAA) | |||||||||
| Arginine | 0.73 | 0.83 |
0.72b |
0.75ab |
0.81a |
0.84 a |
0.015/0.001 | 0.02/0.004 | ns |
| Isoleucine | 0.75 | 0.79 |
0.69b |
0.75ab |
0.80a |
0.83a |
0.016/0.08 | 0.023/0.005 | ns |
| Leucine | 0.81 | 0.76 |
0.72b |
0.77 b |
0.81ab |
0.85 a |
0.01/0.003 | 0.014/0.001 | ns |
| Lysine | 0.78 | 0.80 |
0.75b |
0.78b |
0.80ab |
0.83a |
0.01/0.09 | 0.012/0.001 | ns |
| Met + cystine | 0.77 | 0.79 |
0.70b |
0.76ab |
0.82a |
0.84a |
0.014/0.48 | 0.02/0.001 | ns |
| Histidine | 0.76 | 0.75 |
0.71b |
0.74ab |
0.77ab |
0.79a |
0.14/0.65 | 0.02/0.044 | ns |
| Phenylalanine | 0.75 | 0.81 |
0.72c |
0.78b |
0.81 ab |
0.82 a |
0.01/0.001 | 0.011/0.001 | ns |
| Threonine | 0.68 | 0.80 |
0.66b |
0.74ab |
0.76a |
0.78a |
0.015/0.001 | 0.022/0.006 | ns |
| Tyrosine | 0.78 | 0.75 |
0.69b |
0.77a |
0.79a |
0.82a |
0.01/0.044 | 0.014/0.001 | ns |
| Valine | 0.78 | 0.74 |
0.69b |
0.75ab |
0.78a |
0.81a |
0.015/0.08 | 0.021/0.007 | ns |
| Non-essential amino acid (NEAA) | |||||||||
| Alanine | 0.75 | 0.79 |
0.71b |
0.77a |
0.80a |
0.82a |
0.01/0.001 | 0.012/0.001 | ns |
| Aspartic acid | 0.74 | 0.78 |
0.70c |
0.74bc |
0.79 ab |
0.81a |
0.011/0.015 | 0.015/0.001 | ns |
| Glutamic acid | 0.80 | 0.88 |
0.77c |
0.81b |
0.87ab |
0.89a |
0.012/0.001 | 0.016/0.001 | ns |
| Glycine | 0.70 | 0.79 |
0.70b |
0.74 ab |
0.77 a |
0.79a |
0.011/0.001 | 0.015/0.003 | ns |
| Proline | 0.74 | 0.84 |
0.73 b |
0.77b |
0.81ab |
0.86a |
0.01/0.001 | 0.014/0.001 | ns |
| Serine | 0.68 | 0.76 |
0.64b |
0.73 a |
0.75 a |
0.76 a |
0.012/0.001 | 0.017/0.001 |
ns |
1 See Table 1
* Ileal: ileal digestibility, Exc.: excreta digestibility
Table 10: Ileal and excreta digestibility of amino acids according to model (3)
| Criteria |
Method |
Model | Regression |
||||||
|
Exc.1 |
Ileal1 |
Exc.2 |
Ileal2 |
SD3 |
P |
R2 |
Slope | P- value | |
| Essential amino acids (EAA) | |||||||||
| Arginine | 0.83 | 0.73 | 51.4 | 40.8 | 4.64 | 0.001 | 0.72 | -0.373 | 0.001 |
| Isoleucine | 0.79 | 0.75 | 42.0 | 37.7 | 5.03 | 0.05 | 0.58 | -0.434 | 0.001 |
| Leucine | 0.76 | 0.81 | 44.8 | 49.5 | 3.17 | 0.002 | 0.74 | -0.370 | 0.001 |
| Lysine | 0.80 | 0.78 | 58.1 | 55.9 | 2.69 | 0.15 | 0.63 | -0.249 | 0.01 |
| Met + cys. | 0.79 | 0.78 | 41.6 | 40.1 | 4.37 | 0.40 | 0.62 | -0.437 | 0.001 |
| Histidine | 0.75 | 0.76 | 52.6 | 53.4 | 4.33 | 0.64 | 0.37 | -0.262 | 0.002 |
| Phenylalanine | 0.81 | 0.75 | 54.6 | 49.2 | 2.54 | 0.001 | 0.78 | -0.310 | 0.001 |
| Threonine | 0.80 | 0.68 | 62.4 | 53.9 | 3.44 | 0.001 | 0.66 | -0.201 | 0.008 |
| Tyrosine | 0.75 | 0.78 | 42.1 | 45.2 | 3.32 | 0.03 | 0.71 | -0.388 | 0.001 |
| Valine | 0.74 | 0.78 | 42.6 | 46.7 | 4.52 | 0.04 | 0.56 | -0.369 | 0.001 |
| Non-essential amino acids (NEAA) | |||||||||
| Alanine | 0.79 | 0.75 | 51.3 | 46.7 | 2.83 | 0.001 | 0.75 | -0.332 | 0.001 |
| Aspartic acid | 0.78 | 0.74 | 49.7 | 45.6 | 3.41 | 0.008 | 0.67 | -0.336 | 0.001 |
| Glutamic acid | 0.88 | 0.80 | 51.7 | 43.7 | 3.56 | 0.001 | 0.78 | -0.421 | 0.001 |
| Glycine | 0.79 | 0.70 | 53.9 | 45.4 | 3.29 | 0.001 | 0.76 | -0.297 | 0.001 |
| Proline | 0.84 | 0.74 | 51.0 | 41.3 | 3.22 | 0.001 | 0.83 | -0.385 | 0.001 |
| Serine | 0.76 | 0.68 | 44.2 | 36.2 | 3.99 | 0.001 | 0.72 | -0.373 |
0.001 |
1 Least square means of ileal and excreta digestibility according to model (3)
2 Ileal and excreta digestibility according to extrapolation to 100% SBP
3 SD: standard error
alterations caused by chitin. N retention as a proportion of intake of SBP was estimated at 41 % (Table 8).
Apparent ileal and excreta digestibility of amino acids
The present study showed that digestibility of individual AA in SBP varied between 40% and 60 %. The highest digestibility of EAA was noted for lysine and threonine and lowest for methionine +cystine. Comparisons between the two measurement techniques show that there were differences for 11 of the total of 16 AA determined (p<0.05) between ileal and excreta coefficients, while values for the remainder were not influenced by the site of measurement (p>0.05) (Table 9). Higher excreta digestibility coefficients (p<0.05) were observed for phenylalanine, arginine, alanine, threonine, aspartic acid, glycine, glutamic acid, serine and proline (p<0.05), while higher ileal values were found for tyrosine and leucine (p<0.05).
Analysis according to model (1) demonstrated significant differences between ileal and excreta digestibility for all AA except lysine, methionine +cystine and histidine. The regression equations showed that there was a substantial negative effect for both methods of increasing level of SBP, and model (2) gave no significant difference between the slopes of any AA. Thus model (3) was used to calculate the digestibility of AA in the SBP, as presented in Table 10.
Discussion
The poor digestion of greater CF, ADF, and chitin concentrations by chicken due to high levels of SBP can be used to explain how SBP negatively affects ACTTD (Rahman and Koh, 2018). The outcomes are consistent with research giving chicken diets containing chitin from shrimp meal by Brito et al. (2020ab), Pagala et al. (2021), and Abun et al. (2022). According to Brito et al. (2020a), the amount of diet-related shrimp waste meal the digestibility was reduced. Akter et al. (2019) using 150 g/kg of shrimp waste both CP and DM’s ileal digestibility also decreased (P<0.05) due to the interactions between nutrients and their effects on digestion and absorption. This demonstrates the necessity of including shrimp waste at the appropriate level. It is possible to anticipate that the dietary fiber’s mass will affect peristaltic activity (Jamroz, 2001). Due to the addition of molasses derived from dietary SBP, the OM digestibility was still quite high even though the fraction of rice bran reduced as dietary SBP increased. Additionally, according to Brito et al. (2020b), the dietary element in shrimp meal that decreases digestibility is the ash. Although studies suggest that the component in shrimp that reduces nutrient utilization may be chitin as a naturally occurring N-acetylchito oligosaccharide from crustacean exoskeletons (Khempaka et al., 2006; Brito et al., 2020b).
According to the findings of total digestibility, the addition of the shrimp waste to the whole meal had an additive influence on the amount of nitrogen that could be digested (Brito et al., 2020b). It is likely that the presence of chitin and chitosan in the shrimp is responsible for this result. According to the research, the digestibility of nutrients is enhanced when these components are present. Additionally, the incorporation of shrimp meal into the total diet (Brito et al., 2020b) resulted in a decrease in DM digestibility. This result was probably impacted by the fact that the animals consumed less feed when shrimp meal was present at that level. Despite this, nitrogen digestibility increased along with the amounts of shrimp waste since the meal contained a significant amount of nitrogen. Besides, the nitrogen metabolism in the hindgut may include both the degradation of dietary and non-dietary AA and the synthesis of microbial proteins, which may account for these differences. The equilibrium between the two activities determines whether the recovery of AA in excreta will decrease or increase relative to the ileum. Ammonia can build up if an excessive amount of amino acids that contain nitrogen go through the process of catabolism, which most likely results from the breakdown of amino acids that are out of balance. The ammonia that is created is then transformed into urea, which is carried by the blood to the kidneys so that it can be eliminated through urine. Nitrogen that the body is unable to use in its whole ought to be transformed and then eliminated from the body. In addition, the nitrogen in poultry faeces originates from metabolizable nitrogen, which the body of poultry is unable to absorb (Linh et al., 2022). In the same line Khempaka et al. (2011) showed that chitin in shrimp meal recorded reducing in ammonia production. When AA degradation dominates, the decreased output of amino acids in excreta will lead to an overestimate of amino acid digestibility and vice versa. In the present study, the difference between ileal and total tract digestibility varied in terms of magnitude among diets and individual amino acids. The lower the AA digestibility in the ileum, the greater the amount of AA nitrogen that will reach the hindgut, where it will serve as a substrate for microbial metabolism, resulting in substantial differences between ileal and excreta digestibility. Kadim et al. (2002) stated that in some cases the ileal digestibility was lower, in some cases higher than excreta digestibility and in some cases ileal and excreta digestibility was similar.
The linear decrease in AA digestibility in meals containing SBP may have been due to the increased ADF and chitin levels of SBP. These findings are comparable with those of other researchers (Parson et al., 1983) who discovered that in chicken, a high consumption of dietary fiber increased AA excretion and, thus, decreased AA digestibility. Also, studies with growing pigs fed diets containing dried prawn waste or shrimp by-products indicated that the high chitin content could reduce the total tract digestibility of CP (Mohan and Sivaraman,1993) and Ngoan et al. (2001) found that ileal digestibility of most amino acids was lower in the ensiled shrimp by-product. The current work emphasizes the need to create standard ileal digestibility assays for assessing AA availability in duck feedstuffs. Assays based on ileal digesta have the extra benefit of not being impacted by urinary AA, which may complicate digestibility estimations based on excreta (O’Dell et al., 1960), despite the modest amount of AA discharged in the urine. Another benefit of ileal digesta analysis is that contamination by scurf and feathers, a possible cause of error in excreta analysis, is eliminated.
Conclusion
It can be concluded that the decrease in levels of dietary shrimp by-products leads to the increase in the apparent total tract digestibility of DM, OM, EE and NFE and N retention linearly. The apparent excreta digestibility was greater than the apparent ileal digestibility for the majority of specific amino acids. As dietary levels of shrimp by-products increased, individual amino acid digestibility decreased linearly. The estimated digestibility coefficient of amino acids in shrimp by-products ranged from 40-60%.
acknowledgements
The authors would like to thank the Experiment farm Nam Can Tho for supporting the animals and feeds and Tay Do University for facilitating the experimental implementation.
Conflict of Interest
The authors declared that there were no conflicts of interest.
novelty statement
The research outcomes on amino acid digestibility of crossbred ducks fed shrimp by-product.
authors contribution
1-Nguyen Thi Kim Dong carried out the experiment and wrote the manuscript for publication.
2-Nguyen Van Thu designed and analysed the data of the experiment for publication.
3-Nguyen Hoang Qui handle and fount related refererences for discussing of the manuscript.
References
Abun A., Widjastuti T., Haetami K (2022). Effect of fermented shrimp shell supplementation of low protein diet on the performance of Indonesian native chicken. J. Appl. Anim. Res., 50(1): 612-619. https://doi.org/10.1080/09712119.2022.2123810
Akter M., Graham H., Iji P.A (2019). Response of broiler chickens to diets containing different levels of sodium with or without microbial phytase supplementation. J. Anim. Sci. Technol., 61(2): 87–97 https://doi.org/10.5187/jast.2019.61.2.87
AOAC (2000). AOAC Official Method 994.12 Amino acids in Feeds. In:Horwitz, W. (Eds), Official methods of Analysis of AOAC International, 17th edn. pp 2200. Gaithersburg, MD, USA.
Brito C.O., Junior V.R., Del Vesco A.P., de Castro Tavernari F., Calderano A.A., Silva C.M., de Lima Maciel J.T., de Azevedo M.S.P (2020b). Metabolizable energy and nutrient digestibility of shrimp waste meal obtained from extractive fishing for broilers. Animal Feed Science and Technology, 2020b; 263(2020): 114467. https://doi.org/10.1016/j.anifeedsci.2020.114467
Brito C.O., Silva C.M., Lelis G.R., Corassa A., Velarde J.M.D.S., Silva M.A.S., OliveiraJúnior G.M., Del Vesco A.P., Júnior, V.R. (2020a). Inclusion of shrimp waste meal in diet of free-range chickens. South African J. Anim. Sci., 50(6): 773-778 https://doi.org/10.4314/sajas.v50i6.1.
Foltyn M., Lichovníková M., Rada V., Musilová A (2015). Apparent ileal digestibility of protein and amino acids in protein feedstuffs and trypsin activity in the small intestine in broiler chickens. Czech J. Anim. Sci., 60(8): 375–382. https://doi.org/10.17221/8407-CJAS
Han H.Y., Zhang K.Y., Ding X.M., Bai S.P, Luo Y.H., Wang J.P., Zeng Q.F (2017). Effect of dietary fiber levels on performance, gizzard development, intestinal morphology, and nutrient utilization in meat ducks from 1 to 21 days of age. Poult. Sci., 0:1–9. https://doi.org/10.3382/ps/pex268
Jamroz D., Jakobsen K., Orda J.,Skorupinska J.,Wiliczkiewicz A (2001). Development of the gastrointestinal tract and digestibility of dietary fibre and amino acids in young chickens, ducks and geese fed diets with high amounts of barley. Comparat. Biochem. Physiol. Part A, 130(4): 643-652. https://doi.org/10.1016/S1095-6433(01)00386-5
Kadim I.T., Moughan P.J., Ravindran V (2002). Ileal amino acid digestibility assay for the growing meat chicken comparison of ileal and excreta amino acid digestibility in the chicken. British Poult. Sci. 44(4): 588-597. https://doi.org/10.1080/0007166022000004507
Khempaka S., Chitsatchapong C., Molee W (2011). Effect of chitin and protein constituents in shrimp head meal on growth performance, nutrient digestibility, intestinal microbial populations, volatile fatty acids, and ammonia production in broilers. J. Appl. Poult. Res., 20(1): 1-11. https://doi.org/10.3382/japr.2010-00162
Khempaka S., Koh K., Karasawa Y (2006). Effect of Shrimp Meal on growth performance and digestibility in growing broilers. J. Poult. Sci., 43(3): 250–254. https://doi.org/10.2141/jpsa.43.250
Kim E.J., Utterback P.L., Parsons C.M (2012). Comparison of amino acid digestibility coefficients for soybean meal, canola meal, fish meal, and meat and bone meal among 3 different bioassays. Poult. Sci., 91 :1350–1355. https://doi.org/10.3382/ps.2011-01861
Kumari S., Kishor R. (2020). Chitin and chitosan: origin, properties, and applications. In: Handbook of chitin and chitosan. Volume 1: Preparation and Properties, 1stedn. pp. 1-33. Elsevier. https://doi.org/10.1016/B978-0-12-817970-3.00001-8
Linh N.T., Dong N.T.K., Thu N.V (2022). Effect of dietary lysine and energy levels on apparent nutrient, nitrogen, and amino acids digestibility of local Muscovy ducks. Adv. Anim. Vet. Sci., 10(2): 253-262. https://doi.org/10.17582/journal.aavs/2022/10.2.253.262
Mao X., Guo N., Sun J., Xue C (2017). Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Cleaner Prod., 143(2017):814–823. https://doi.org/10.1016/j.jclepro.2016.12.042
Mohan S.K.M.,Siravaman E (1993). The feeding value of dried prawn waste in the rations for swine. J. Vet. Anim. Sci., 24: 103-108.
Ngoan L.D., Ogle B. Lindberg J.E. (2000). Ensiling techniques for shrimp by-products and their nutritive value. Asian-Australasian J. Anim. Sci., 13(9): 1278-1284. https://doi.org/10.5713/ajas.2000.1278
O’Dell B.L., Woods W.D.,Laerdal O.A., Jeffay A.M., Savage J.E. (1960). Distribution of the major nitrogenous compounds and amino acids in chicken urine. Poult. Sci. , 39(2): 426- 432. https://doi.org/10.3382/ps.0390426
Pagala M.A., Saragih F.R., Salido W.L., Isnaeni P.D., Napirah A (2022). The Effect of Fish, Shrimp Head, and Crab Shell Meal in Different Proportions on Carcass, Liver, and Abdominal Fat Percentage of Super Native Chicken. Adv. Biolog. Sci. Res., 20 (1): 184-186 https://doi.org/10.2991/absr.k.220309.038
Parsons C.M., Potter L.M., Brown Jr,R.D., Wilkins T.D., Bliss B.A. (1982). Microbial contribution to dry matter and amino acid content of poultry excreta. Poult. Sci., 61(5): 925-932. https://doi.org/10.3382/ps.0610925
Rahman M., Koh K. (2018). The effects of formic acid-treated shrimp meal on laying performance and egg quality in aged laying hens. J. Appl. Poult. Res., 27(1): 45-50. https://doi.org/10.3382/japr/pfx038
Ravindran V., Hew L.I., Ravindran G., Bryden W.L. (1999). A comparison of ilealdigesta and excreta analysis for the determination of amino acid digestibility in food ingredients for poultry. Brit. Poult. Sci. 40(2): 266-274. https://doi.org/10.1080/00071669987692
Rose S.P. (1997). Principles of Poultry Science. CAB International, Wallingford, UK.
Takemasa, M (1992). Improvement of the method for chromic oxide determination with potassium phosphate reagent. Annual Res. Rep. N. I. A. I. 52: 7-13.






