Advances in Animal and Veterinary Sciences
Short Communication
Development of Real-Time PCR Assays for Detecting Matrix Metalloproteinases-2 & 9 Over-expression in Canine Mammary Tumours
Sonal Saxena1*, Sameer Shrivastava1, Richa Arora1, Shahid Hussain1, Subas Chandra Jena1, Manoj Kumar1, Resma Kochumanayin Vasu1, Saumya Srivastava1, Priyanka Sharma1, Naveen Kumar2, Kuldeep Dhama3
1Division of Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar, U.P., India; 2Division of Veterinary Surgery, Indian Veterinary Research Institute, Izatnagar, U.P., India; 3Division of Pathology, Indian Veterinary Research Institute, Izatnagar, U.P., India.
Abstract | Matrix Metalloproteinases (MMP) 2 & 9 are overexpressed in wide variety of cancers and play important roles in tumour cell proliferation, invasion and metastasis. Tissue and serum levels of MMP2 and MMP9 correlate with disease prognosis. Real-time PCR is emerging as an alternative or supplementary technique to immunohistochemistry (IHC) for sensitive detection of tumour markers in tissues. Therefore, in this study real-time PCR assays were standardized for detecting over-expression of MMP2 and MMP9 in canine mammary tumour (CMT). Primers designed for real-time PCR analysis of MMP2 & MMP9 were specific as indicated by amplification plots and melt-curve analysis of amplicons. These amplicons showed sharply defined melting curves with single peaks at expected melting points. The over-expression of MMP2 and MMP9 was examined in 12 CMT tissues by real-time PCR analysis. Over-expression of MMP2 & MMP9 was observed in 58.3(7/12) and 41.6 % (5/12) cases of CMT respectively. Expression levels of MMP2 were 2.23±0.10 to 29.80±3.5 fold higher and for MMP2, 1.5±0.56 to 22.67±2.35 fold higher in cases of CMT than normal mammary gland biopsy. Approximately 33.3 % (4/12) of CMT tissues showed co-expression of both MMP2 and MMP9. Further studies are required to correlate MMP2 and MMP9 expression levels with CMT prognosis.
Keywords | MMP2, MMP9, Matrix-metalloproteinase, Real-time PCR, Canine mammary tumour (CMT)
Editor | Yashpal S. Malik, Indian Veterinary Research Institute (IVRI), Izatnagar 243122, Bareilly, Uttar Pradesh, India.
Received | June 20, 2016; Accepted | June 27, 2016; Published | July 01, 2016
*Correspondence | Sonal Saxena, Division of Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar, U.P., India; Email: sonalvet@gmail.com
Citation | Saxena S, Shrivastava S, Arora R, Hussain S, Jena SC, Kumar M, Vasu RK, Srivastava S, Sharma P, Kumar N, Dhama K (2016). Development of Real-Time PCR assays for detecting Matrix Metalloproteinases-2 & 9 over-expression in canine mammary tumours Adv. Anim. Vet. Sci. 4(7): 342-345.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.7.342.345
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Saxena et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Matrix metalloproteinases (MMPs) encompasses a family of secreted, calcium dependent, zinc containing endopeptidases. These enzymes are responsible for tissue remodelling and are capable of degrading all kinds of extracellular matrix proteins including collagens, matrix glycoproteins, gelatin, elastins, and proteoglycans. MMPs are involved in regulation of cell behaviours like cellular differentiation, proliferation, angiogenesis, invasion, and apoptosis. Thus, these enzymes have multi-factorial roles during tumor progression: promoting establishment, tumor cell exfoliation, invasion and angiogenesis. The degradation of extracellular matrix components is crucial for tumor cell invasion and MMPs, especially MMP2 (72 kDa gelatinase A) and MMP9 (92 kDa gelatinase B), play critical role in degradation of gelatin and type IV collagen, the two major constituents of extracellular matrix. MMP9 and MMP2 are involved in invasion and metastases of many human cancers such as cancers of colon (Karakiulakis et al., 1997), breast (Bachmeier et al., 2001), brain tumours (Pagenstecher et al., 2001), prostate cancer (Brehmer et al., 2003), cervical cancer (Sato et al., 2004), melanoma (Bérubé et al., 2005) and many others. Further these gelatinases are also overexpressed in canine cancers such as canine mammary cancers, canine osteosarcoma and cutaneous mast cell tumors (Loukopoulos et al., 2003; Lana et al., 2000; Krupakaran et al., 2013). Studies have shown that tissue and serum level of MMP9 and MMP2 strongly correlate with disease prognosis (Li et al., 2004; Puzovic et al., 2014). Therefore, determination of MMP2 and MMP9 levels may help clinicians for determining prognosis and response to treatment. Currently gelatin zymography and immunohistochemistry (IHC) are used widely to determine expression levels of MMPs. Although, these are robust techniques, but they are time-consuming and labour intensive. Further, IHC scores are subjected to inter- and intra-observational variability (Lawrie et al., 2012). Nowadays, real-time PCR has emerged as an alternative or supplementary technique to IHC for sensitive detection of tissue markers (Gjerdrum et al., 2004; Teixidó et al., 2014). Therefore, this study was taken up to standardize real-time PCR assays for detecting over-expression of MMP2 and MMP9 in cases of canine mammary tumour (CMT), an important neoplastic condition of female dogs accounting for more than 40% of tumours diagnosed (Sleeckx et al., 2011).
The tissue samples (n=12) used in the study were obtained from clinical cases of canine mammary tumours (CMT) referred for surgery to “Referral Veterinary Polyclinic”, Indian Veterinary Research Institute (IVRI), Izatnagar, Bareilly, India. The tissues were confirmed as tumour tissues upon histological examination of H & E stained tissue sections according to World Health Organization (WHO) criteria for CMTs (Misdorp et al., 1999).
Total RNA was extracted from tumour tissues preserved in RNAlater reagent (Qiagen, Hilden, Germany) using RNeasyplus™ mini kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions. The RNA was quantified using Qubit RNA BR Assay Kit (Invitrogen, USA) and the integrity of RNA was confirmed by Experion RNA StdSens Analysis Kit (Bio-Rad) using “Experion Automated Electrophoresis System” (Bio-Rad, Hercules, USA). The purity of RNA was assessed by calculating OD260/OD280 using Nanodrop machine (NanoDrop1000, Thermo Scientific, Singapore). The cDNA was then prepared from total RNA using cDNA synthesis kit (Fermentas, USA) as per the manufacturer’s instructions. The cDNA from each tumour sample was synthesized using Oligo (dT)18 primers and 1 μg total RNA in a total volume of 20 µl. The prepared cDNA was stored at -80ºC for downstream applications.
The expression of MMP2 & MMP9 mRNA was assayed using Applied Biosystems® 7500 fast real-time PCR system. A set of intron-spanning primer sequences (Table 1) for dog MMP2 & MMP9 genes were designed using Premier 5.0 software (National Bioscience) and analyzed using oligoanalyzer 3.1. Ribosomal Protein S 19 (RPS19) gene was selected as an endogenous control to normalize the amount of input RNA or cDNA. The reaction was performed using KAPA SYBR FAST qPCR master mix (KAPA Biosystems, Boston, MA, USA) as per the manufacturer’s instructions. Briefly, 10µl of the reaction mixture, consisting of 5µL SYBR FAST qPCR master mix, 0.4µl cDNA and 200 nmol/L of forward and reverse primers, was subjected to initial denaturation at 95 ºC for 3 min followed by 40 cycles of denaturation at 95 ºC for 3 s, and annealing/extension at 60 ºC for 20s. Optical data was analysed using ABI 7500 data analysis software. The PCR threshold cycle (Ct), defined as the cycle number at which the fluorescence reaches 10 times the standard deviation (SD) of the baseline, was determined by the software. Average Ct for the samples was calculated by the software. The relative gene expression levels were determined using the 2-ΔΔCt method (Schmittgen and Livak, 2008). All mRNA expression levels were normalized to that of normal mammary tissue obtained from dog post mortem case. Data were expressed as the mean values calculated from experiments performed in triplicate. The specificities of the PCR amplicons were confirmed using melting curve analysis.
The OD260/280 of the RNA samples isolated from CMT tissues was found to be ~1.9 indicating good purity of the RNA samples. The cDNA’s synthesized from the RNA samples were used for analysis of MMP2 and MMP9 gene over-expression by real-time PCR. Over-expression of MMP9 was detected in 5 out of 12(50%) tissues of canine mammary tumour. The relative expression levels were determined in comparison to normal mammary gland biopsy obtained from of a female dog post-mortem case. The RPS19 gene was used as a reference gene or endogenous control for normalization of data. The amplification curves for MMP9 real-time PCR analysis are illustrated in Figure 1A. Single and sharply defined melting curves with narrow peaks were obtained showing specificity of real-time PCR amplification (Figure 1B).The expression levels of MMP9 were 1.5±0.56 fold to 22.67±2.35 fold higher in CMT tissues than the normal mammary gland tissue.
Table 1: Details of primes used for real-time PCR analysis
|
S.No |
Gene |
Forward Primer (5’-3’) |
Reverse primer ( 5’-3’) |
|
1. |
MMP2 |
GGGACAAGAACCAGATCACATAC |
GTGGATACGAGAAAACCGCAG’ |
|
2. |
MMP9 |
CGCATGACATCTTCCAGTACCA |
CCGAGAATTCACACGCCAGTA |
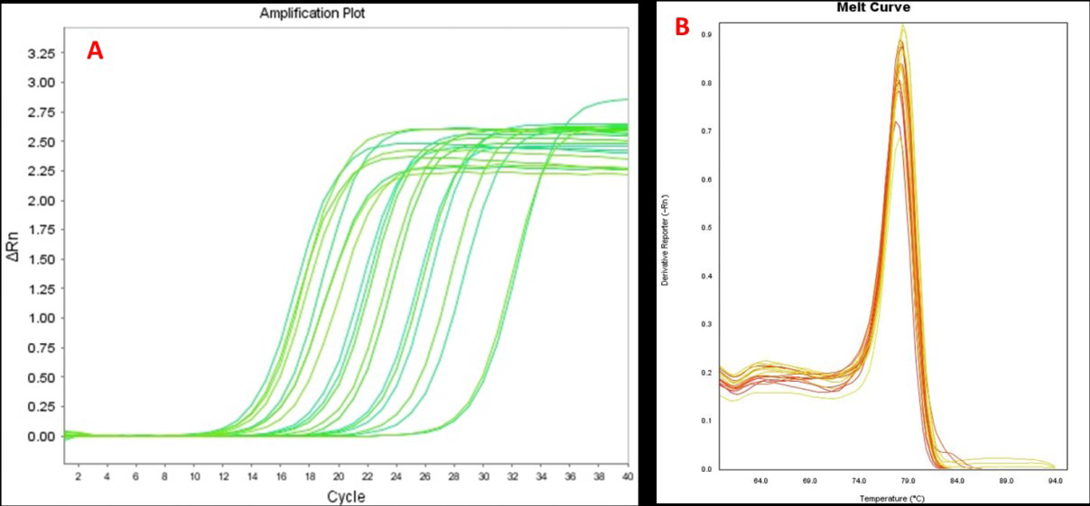
Figure 1: Amplification plots (A) and melting curves (B) for MMP9 gene real-time PCR analysis in CMT tissues
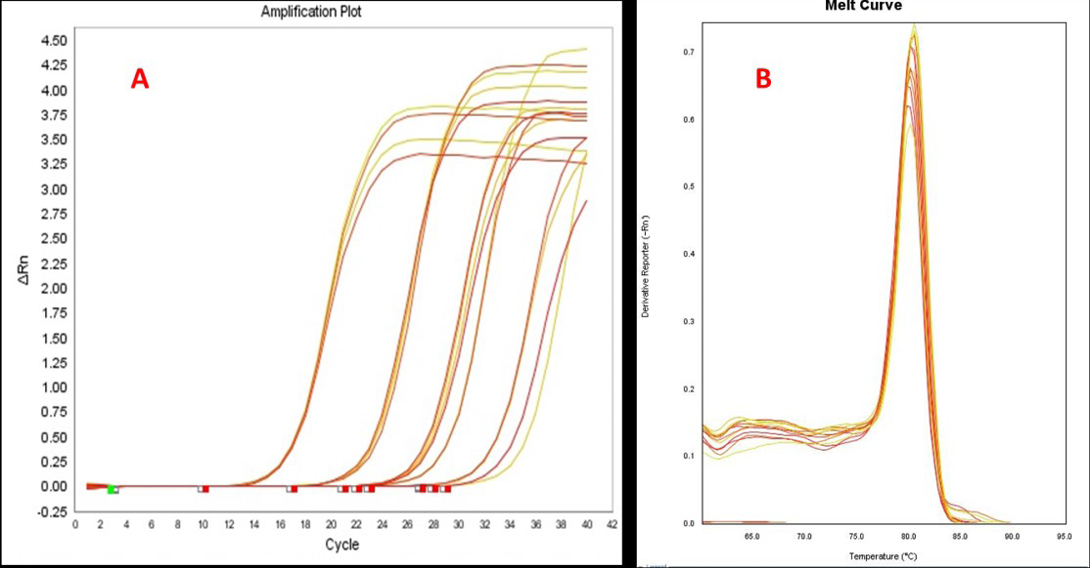
Figure 2: Amplification plots (A) and melting curves (B) for MMP2 gene real-time PCR analysis in CMT tissues
Table 2: Real-time PCR analysis for MMP2 & MMP9 gene over-expression in CMT tissues
|
Gene |
%age of tissues showing over expression of MMP2/MMP9 |
Relative MMP2/MMP9 expression levels (relative to normal dog mammary gland |
|
MMP9 |
41.6% (5/12) |
1.5±0.56 to 22.67±2.35 fold |
|
MMP2 |
58.3 (7/12) |
2.23±0.10 to 29.80±3.5 fold |
|
MMP2 & MMP9 both |
33.3%(4/12) |
4.5±0.21 to 52.47±5.8 fold |
Over-expression of MMP2 gene was detected in 7 out of 12 (58.3%) CMT tissues examined by real-time PCR. The samples from CMT cases showed 2.23±0.10 to 29.80±3.5 fold higher expression levels of MMP2 as compared to normal healthy mammary gland tissue of dog. The amplification plots for real-time PCR assay for MMP2 gene expression in CMT tissues are illustrated in Figure 2A. Specificity of real-time PCR assay was ascertained by melt curve analysis (Figure 2B). Out of 12 CMT tissues, 4 CMT tissues showed co-expression of MMP2 and MMP9. Data for real-time PCR analysis of MMP2 and MMP9 over-expression in CMT tissues is compiled in Table 2.
Acknowledgements
The authors are thankful to Director, IVRI, and Izatnagar for providing necessary infrastructure and facilities to carry out this research work. Authors are also thankful to Department of Biotechnology (DBT), Govt. of India (Project grant No. BT/PR13900/ADV/57/44/2010) and Indian Council of Agricultural Research (ICAR) NAE programme on biosensors for supporting the study by research grants.
Conflict of Interests
Authors of this manuscript declare no conflict of interest.
authors’ contribution
S. Saxena & S. Shrivastava designed the work, conducted real-time PCR experiments and drafted the manuscript. R. Arora, M. Kumar, R.K. Vasu & S. Srivastava isolated RNA from tumour tissues, prepared cDNA and helped in standardization of real time PCR. S. C. Jena, and P. Sharma helped in data analysis. N. Kumar collected tumour tissues from surgically operated CMT cases. K. Dhama helped in data analysis and drafting the manuscript.
References






