Advances in Animal and Veterinary Sciences
Short Communication
Survey of Hepatic Lesions and Incriminating Pathogens in Rural Scavenger Chickens in Maiduguri, North-Eastern Nigeria
Halima Idrissa Gambo1, Asinamai Athliamai Bitrus1*, Babagana Mohammed Adam1, Dauda Mohammed Goni2, Iliya Dauda Kwoji2, Solomon Jauro Thliza2, Peter Anjili Mshelia3
1Department of Veterinary Pathology, Faculty of Veterinary Medicine, Maiduguri, Borno, Nigeria; 2Department of Veterinary Microbiology and Parasitology, Faculty of Veterinary Medicine, Maiduguri, Borno, Nigeria; 3Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, University of Maiduguri, P.M.B 1069 Maiduguri, Borno State, Nigeria.
Abstract | The survey of hepatic lesions in rural scavenger chickens was carried out in Maiduguri live chicken market. A total of 100 liver tissue and swab samples were collected for histopathological and bacteriological analysis. The result obtained revealed that 20 % of the liver were enlarged, 57% have variable degree of hemorrhage while 77% were pale. In addition, friability and fibrin deposits were observed in 3% and 5% of the liver respectively. Similarly, histopathologic lesions observed, includes varying degree of severe congestion of the central veins, severe mononuclear cellular infiltration around the portal triad, necrosis and degeneration of the hepatocytes. Bacteriological analysis revealed that out of the hundred liver swabs collected, 77 showed positive growth. However, 63 out of the 77 bacteria isolated showed mixed growth while the remaining 14 isolates showed single growths on both MacConkey and blood agar respectively. In addition, out of the 115 bacteria isolated, 37 (32.2%) were Enterococcus specie, 27 (23.5%) were Escherichia coli, 29 (25.2%) were Corynebacteria specie while 15 (13%) and 7(6.1%) of the isolates were Bacillus subtilis and Staphylococcus aureus respectively. Varying degrees of hepatic lesions and incriminating pathogens were observed in all liver samples.
Keywords | Chickens, Hepatic, Lesions, Poultry, Rural, Scavenger
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 09, 2016; Revised | February 26, 2016; Accepted | March 04, 2015; Published | March 18, 2016
*Correspondence | Asinamai Athliamai Bitrus, Department of Veterinary Pathology, Faculty of Veterinary Medicine, Maiduguri, Borno State, Nigeria; Email: abasinamai@gmail.com
Citation | Gambo HI, Bitrus AA, Adam BM, Goni DM, Kwoji ID, Thliza SJ, Mshelia PA (2016). Survey of hepatic lesions and incriminating pathogens in rural scavenger chickens in Maiduguri, North-Eastern Nigeria. Adv. Anim. Vet. Sci. 4(4): 174-177.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.4.174.177
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Gambo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Poultry production in Nigeria represent a very important national resources which contributes substantially to national economic growth and supplied the general population with the much needed high animal protein required for healthy living (Oladeebo and Ambe-Lamidi, 2007). Nigeria has the largest poultry population in Africa with an estimated population of about 137 million chickens. The total poultry production in Nigeria was estimated to be between 133-165 million (Akinwumi et al., 1979). However, there is a consensus that about 90% of this figure is derived from the rural poultry stocks which in turn is composed of chickens (91%), guinea fowl (4%), ducks (3%), turkeys and others (2%).
Rural poultry production is an important agricultural activity of almost all rural community in Africa (Ambali et al., 2003). Estimation of the livestock population in Africa revealed that poultry population is high (Ambali et al., 2003). In addition, studies have shown that about 80% of these poultry were concentrated in the rural areas under free range system. Hence, serving as an important sources of animal protein in the form of meat and egg and in addition, a reliable source of income (Alders and Spadbrow, 2001). Furthermore, it is difficult to assign monetary value with regards to the role of rural scavenger chicken as an agent of pest control and for traditional ceremony and festival.
Rural poultry have been reported to play a very significant role to national economy and an important source of income to many small communities (Ambali et al., 2003). However, production levels have been observed to have fallen below desirable limits as a result of poor management practice.
It is widely believed that rural chickens act as a potential reservoir of infection. In addition, a number of investigations have reported the importance of poultry diseases among indigenous chicken in Africa. Little research has been done on rural poultry health in Maiduguri. In addition, it is believed that disease and poor management practice are regarded as the factor limiting the poultry production in Africa. This study was designed to survey the hepatic lesions in rural scavenger chickens and associated pathogens with a view to understanding the health status of rural chicken slaughtered at Maiduguri live chicken market.
A total of 100 liver samples were collected from the slaughter slab of the Maiduguri live chicken market immediately after processing. Liver samples were examined for gross lesions. Gross changes were noted and photographed with a camera. Tissue samples were collected for histopathology. In addition, a corresponding 100 swabs were aseptically taken from the liver using sterile swab stick for bacteriological assay.
Media used for the purpose of this research includes: Tryptic soy broth (TSB), Blood agar (BA), Mannitol salt agar (MSA), MacConkey agar (MA) and Eosin methylene blue agar (EMB) (Oxoid, Basingstoke, UK). All media were prepared aseptically according to the manufacturers’ instruction and autoclaved at 121oC I bar for 15 minutes. Swab samples were enriched in a tryptic soy broth (Oxoid, UK) and incubated aerobically at 37oC for 18- 48 hours. Broth were cultured overnight on a freshly prepared blood and MacConkey agar (Oxoid, UK) respectively. Identification of S. aureus was carried out using colony morphology, gram staining, mannitol fermentation, and catalase and tube coagulase test as described by Aklilu et al. (2010). While E. coli identification was carried out using Eosin methylene blue agar, Triple sugar iron (TSI), sulphide indole motility test (SIM), citrate test, urease test and methyl Red and voges- prauskeur test (MR-VP) as described by Smith and Scotland (1993). Similarly, identification of Enterococcus species, Corynebacteria species and Bacillus subtilis were carried out according to methods described by Teo and Tan (2005), Valenzuela et al. (2010) and Watts et al. (2000) respectively.
Tissues samples obtained from a cut section of the liver were subjected for routine histopathological analysis as described by Preman (2013). Processed Tissue were embedded in paraffin and 4µm thick sections were produced using a microtome while sections were stained using hematoxylin and eosin (H&E).
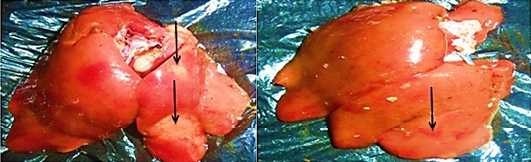
The distribution of gross and histologic lesions observed from liver samples collected at the point of slaughter in Maiduguri live chicken market revealed a variable degree of identifiable lesions which includes; 20% enlargement, 57% hemorrhage and 77% paleness. In addition, friability and fibrin deposits were also observed in 3% and 5 % of the liver samples respectively (Table 1). In addition, a varying degree of severe congestion of central veins, severe mononuclear cellular infiltration around the portal triads, necrosis and degeneration of hepatocytes were also observed (Figure 1 and 2).
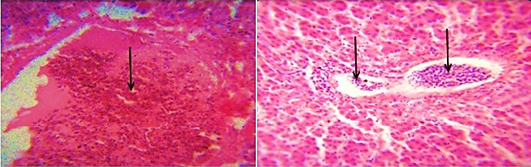
Figure 1: Photomicrograph of the avian liver showing severe congestion of the central vein (arrows) H&E x400 and x800
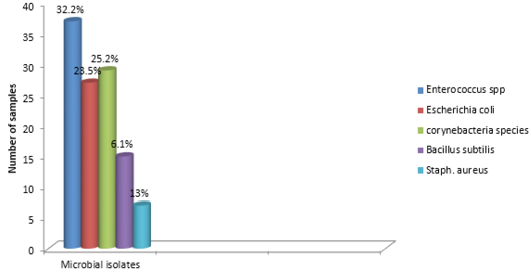
The findings of the bacteriological isolation and identification of the isolates revealed that 77 out of the 100 liver swabs collected were positive. However, 63 (81.8%) of the isolates showed mixed growth while 14 (18.18%) showed single growth on blood and MacConkey agar respectively. In addition, phenotypic and biochemical identification of the isolates revealed that 37 (32.2%) of the isolates were Enterococcus specie while 27 (23.5%) and 29 (25.2%) were Escherichia coli and Corynebacteria (Figure 3). In addition, 15 (13%) and 7 (6.1%) of the isolates were Bacillus subtilis and Staphylococcus aureus 7 (6.1%) respectively (Table 1).
The role of poultry production to the development of the socio-economic status of a nation cannot be underestimated. Poultry production have proven to be a major profession for small scale farm holders and a significant impetus to national economic growth (Adebayo and Adeola, 2005). In many developing countries of the world, apart from serving as a source of animal protein, poultry production serves as a major source of income to many homes in the rural communities and in some instances urban centres (Ambali et al., 2003). There is therefore the need to ensure the maintenance of good hygienic measures in order to guard against the negative impact of disease and mortality in poultry production. The findings of this research study obtained revealed the appearance of three major gross and histopathologic lesions which includes paleness, hemorrhages and enlargement. Severe congestion of the central vein, mononuclear cellular infiltration around the preportal triads, necrosis and degeneration of the hepatocytes. Other lesions observed include friability and fibrin deposits. The appearance of these lesion in almost all liver tissues collected is a direct reflection of the management system. This is because in Maiduguri, rural poultry production system is mainly free range and only in few cases of semi intensive system. This however, may lead to exposure of birds to a variety of pathogens. In addition, while some of the birds may die as a result of the exposure, others may recover and serve as reservoirs of infection. On the other hand, since most rural poultry farmers do not have access to good veterinary services, the probability of the birds coming down with disease is high. The implication of this finding is that, huge economic losses are incurred.
Table 1: Distribution of Gross and Histopathologic lesions as well as incriminating microbial pathogens
|
Lesions |
Microbial Isolates |
|||
|
Gross |
% |
Histopathologic |
% |
|
|
Enlargement |
20 |
Severe congestion |
E. coli |
23.50 |
|
Hemorrhage |
57 |
Severe mononuclear cellular infiltration |
Enterobacteria species |
33.20 |
|
Paleness |
77 |
Cellular degeneration |
Corynebacteria species |
25.20 |
|
Friability |
03 |
Necrosis |
Bacillus subtilis |
13.0 |
|
Fibrin deposits |
05 |
- |
Staphylococcus aureus |
6.10 |
Bacteriological analysis of the incriminating pathogens isolated from the liver samples revealed enterococcus species as the most predominant isolates (32.2%). This is followed by Escherichia coli (23.5%) and Corynebacteria species (25.2%). In addition, S. aureus and Bacillus subtilis were observed in 6.1% and 13% respectively. These pathogens are commonly found inhabiting the environment and gastrointestinal tracts of animals. Thus can be easily associated with poultry disease since rural chickens are known to freely scavenge in the environment (Alders and Spadbrow, 2001). Furthermore, the isolation of these pathogens from the liver may not necessarily imply that the birds were infected prior to slaughter. However, this can be due to contamination arising from the water used in washing the carcass, contamination of the slaughter slab or directly from the hand of poultry meat handlers. For instance, Escherichia coli is known to be widely spread commensal bacteria inhabiting the gastrointestinal tract of humans and animals and most pathogenic strains are of veterinary and public health significance (Costa et al., 2008). In addition, studies have also revealed that strains of E. coli can be isolated within 40 feet radius outside the poultry house (Davis and Morishita, 2005). Thus, indicating that the reason why the pathogen is considered as a major contaminant in poultry processing plant (Jimenez et al., 2003).The findings further provides substantial evidence as to the reason why E. coli is the most predominantly isolated bacteria obtained from the liver samples. Furthermore, the presence of the isolated pathogens can also be due to carcass contamination or the presence of ongoing infection prior to slaughter. In addition, bacteria belonging to the Enterobacteriaceae group have been reported as a serious problem to meat processing plants (Adetunji and Odetokun, 2011). Contamination of poultry meat can occur when there is rupture of the intestine, carcass contamination by equipment as well as by handlers during packaging (Jackson et al., 2001).
The isolation of S. aureus from liver of chickens is a classic case of contamination. This can either be through the environment, birds’ feces or from the poultry meat handlers (Valenzuela et al., 2010). In addition, S. aureus considered as an emerging zoonosis and one of the most important causes of food borne infection worldwide (Chiu et al., 2012; Saleha and Zunita, 2010). Thus, we can infer that their presence in the liver could be the result of contamination of the slaughter slab and not due to infection. In either case however, they can predispose to live threatening infection in both humans and animals. Because they have the potentials to rapidly acquire resistance and virulence determinants.
In conclusion, it was observed that majority of the liver samples obtained from rural chickens slaughtered at the Maiduguri live chicken market harbours a varying degree of gross and histopathologic lesions. We therefore recommend the adherence of strict hygienic measures at all levels of rural poultry production chain and to ensure the slaughter of apparently healthy birds with a view to militating against the spread of pathogenic bacteria which may be detrimental to health.
Acknowledgement
The authors wish to thank all the laboratory staff of Medicine laboratory, Department of Veterinary Medicine, Faculty of Veterinary Medicine, University of Maiduguri, for their kind cooperation throughout the course of this study. The authors also wish to thank the management of the Maiduguri live chicken market, Maiduguri, Borno State.
Conflict of interest
No any conflict of interest.
Author’s contribution
The design and execution of this research study is a collective effort of all the authors. All authors were also involved in the critical analysis and review of the manuscript.
Reference