Advances in Animal and Veterinary Sciences
Research Article
Effect of Robusta Coffee from Lampung (Coffea canephora) to Relative Number of Ho-1, Nrf2 and Duodenum Tissue Histopathology in Chicken
Djalal Rosyidi1, Dahliatul Qosimah2*, Indah Amalia Amri2, Dodik Prasetyo3, Fajar Shodiq Permata4, Agri Kaltaria Anisa5, Lucky Retno Putri2, Nanda Ayu Cindyasputri2, Wulandari2, Yohana Leuricha2, Lilik Eka Radiati1
1Laboratory of Animal Product Technology, Faculty of Animal Husbandry, Universitas Brawijaya, Indonesia; 2Laboratory of Microbiology Dan Immunology, Faculty of Veterinary Medicine, Universitas Brawijaya, Universitas Brawijaya, Indonesia; 3Clinical of Animal, Faculty of Veterinary Medicine, Universitas Brawijaya, Universitas Brawijaya, Indonesia; 4Laboratory of Pathology Anatomy, Faculty of Veterinary Medicine, Universitas Brawijaya, Universitas Brawijaya, Indonesia; 5Laboratory of Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya.
Abstract | Coffee contains high phenols, especially chlorogenic acid which acts as a modulation of the immune response, and antioxidants. Robusta coffee (Coffea canephora) is a type of coffee with high levels of chlorogenic acid and high caffeine content which has the potential as an antioxidant so that it can be used as a green coffee extract (GCE). Green coffee extract is made from roasted coffee beans. This study used 48 layer chickens, consisting of 4 treatments and 12 replications consisting of negative controls (healthy chickens without coffee supplementation), T1 (coffee extract dose 500 mg / kg BW), T2 (coffee extract dose 1000 mg / kg BW) and T3 (coffee extract dose 1500 mg / kg body weight. Data in the form of quantitative duodenum organ histopathology using haematoxylin and eosin staining and histopathological picture of duodenum were observed using a microscope and analyzed descriptively, test for Nrf2 and HO-1 with flow cytometri the result of robusta coffee (Coffea canephora) extract from lampung had a good effect at a dose of 500 mg / kg body weight against the relative number of Nrf2, HO-1, and histopathological improvement in the duodenum. The conclusion of this study shows that robusta coffe e (Coffea canephora) extract has the potential as an anti-inflammatory and antioxidant in chickens.
Keywords | Robusta coffee, Intestine, Antioxidant, Green coffee, Inflammation
Received | September 27, 2019; Accepted | March 12, 2020; Published | March 25, 2020
*Correspondence | Djalal Rosyidi, Laboratory of Animal Product Technology, Faculty of Animal Husbandry, Universitas Brawijaya, Indonesia; Email: djalal_tht@ub.ac.id
Citation | Rosyidi D, Qosimah D, Amri IA, Prasetyo D, Permata FS, Anisa AK, Putri LR, Cindyasputri NA, Wulandari, Leuricha Y, Radiati LE (2020). Effect of robusta coffee from lampung (Coffea canephora) to relative number of Ho-1, Nrf2 and duodenum tissue histopathology in chicken. Adv. Anim. Vet. Sci. 8(4): 422-427.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.4.422.427
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Rosyidi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Coffee is one of the export commodities and sources of foreign exchange from 12 leading commodities included in the 2015-2019 national strategic plan in Indonesia (Dirjenbun, 2015). Coffee beans have benefits as an antioxidant, antiobesity, hepatoprotective and immunostimulator (Haque et al., 2013). Coffee has the largest active content of phenol 1300-3700 mg in 100 grams of coffee (Kreicbergs et al., 2011) with the main class of chlorogenic acid (CGA) (Farah, 2012) as much as 4-14% (Nallamutu et al., 2015) which functions as an immunomodulator, antioxidant and anti-inflammatory. In addition, coffee can reduce proinflammatory cytokines in humans but the presence of chlorogenic acids is limited by bioavability and low stability in the intestine (Nallamutu et al., 2015). The content of robusta coffee beans is caffeine content of 2%, essential oils 10-16%, chlorogenic acid 6-10%, sugar 4%, cellulose 22-27%, polyphenols 0.2% (James and Spillane, 1990). Based on the research of Belitz et al., 2009, the content of chlorogenic acid in Robusta coffee was 7.1-12.1% higher than chlorogenic acid in Arabica coffee at 6.7-9.2%. Until now there has been no scientific evidence of the role of coffee as an anti-inflammatory and antioxidant in poultry, especially chicken. So this study aims to determine the effect of Robusta extract from Lampung as an anti-inflammatory and antioxidant in chickens.
MATERIAL AND METHODS
This study used ISA brown strain layer chickens aged 1 day and has obtained Ethical Clearance No. 1142-KEP-UB
Making coffee extract, phytochemical and lcms (liquid chromatography–mass spectrometry) test
The coffee extraction was carried out at UPT Materia Medika, Batu, Indonesia using 90% ethanol solvent. 414 grams of Lampung Robusta coffee was soaked with 1500 ml of 90% ethanol in a jar then closed tightly and shaked using a laboratory shaker at 50 rpm. The liquid extract was filtered and evaporated using a Rotatory Evaporator. The results of extraction in the form of brown liquid are used for research.
Pereparation of animals
The study used one day old layer chicken consisting of 4 treatment groups with 12 replications. One day old layer chicken strain Isa Brown, adapted and then vaccinated with the ND, AI, and gumboro vaccine. The treatment consisted of negative controls (healthy chickens without coffee), T1 (coffee extract dose of 500 mg / kg BW), T2 (coffee extract dose 1000 mg / kg BW) and T3 (coffee extract dose 1500 mg / kg BW). Coffee extract was given for 14 days, on days 3-16.
Nrf2 and HO-1 examination
Spleens that have been collected on day 20, were crushed using mortar and PBS was added to make a suspension. The suspension fluid was taken and ready to be checked using flow cytometry with the following procedure: (i) The specimen was pipetted into the falcon tube as much as 50 μL. (ii) Added 10 pL of Anti-Nrf2 FITC and Anti-HO-1 FITC reagents. (iii) Mixed homogeny on vortex mixer, then incubated 15 minutes 20-25 °C in a dark room. (iv) Diluted 50 p.L of I Ox FACS pelysis solution with 450 p.L of distilled water and then mixed homogeneously. (v) After the incubation time was complete, the sample was added 450 рL of diluted FACS (lx) reagents. (vi) Mixed homogeny, then incubated 15 minutes 20-25 ° C in a dark room. (vii) After the incubation period was completed, an analysis was done using the FACS tool.
Histopathology examination
Chickens were subjected to necropsy by veterinarian. The duodenum was then removed and subjected to histopathological examination using Hematoxylin eosin staining (Islam et al., 2006). The duodenal histhopathology was examined using an Olympus CX31 microscope coupled with a Micro computer integrated digital imaging analysis system. Villus height was measured from the tip (lamina propira) from villi to the base (villus crypt junction) (Nasir et.al, 2013). The height of the villi was measured using Image Raster computer program.
Data analysis
Histopathological data of duodenum is in the form of descriptive qualitative to describe changes of the duodenal epithelium . As for the quantitative Nrf2 and HO-1, the data obtained was further analyzed using the ANOVA test with a confidence level of 95%.
RESULT
Active content of robusta coffee extract (Coffea canephora) lampung
The active content of Lampung coffee extract using phytochemical tests showed the presence of tannins and alkaloids (Ezeonu and Chigozie, 2016). LC-MS test results show ed the presence of Chlorogenic acid (CGA).
Relative amount of HO-1
The results showed that on the relative amount of HO-1, the negative control had significantly higher amount (notation a) compared to the treatment groups, which all had comparable results (notation b). The T1 group showed the average of relative amount of HO-1 higher than the other treatment groups (Figure 1).
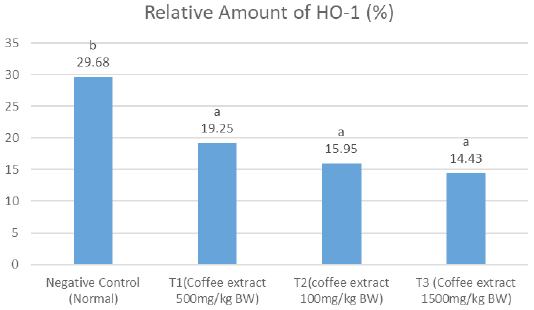
Figure 1: Notation differences (a and b) show a significant difference between treatments for the relative level of HO-1 (P <0.05).
Relative number of Nrf2
The results showed that on the relative number of NRf2, the negative control had significantly higher amount (notation c) compared to the treatment groups, which all had comparable results (T1 and T had notation b, while T3 had notation c) (Figure 2). The average of T1 treatment showed a higher Nrf2 relative number compared to the other treatment groups.
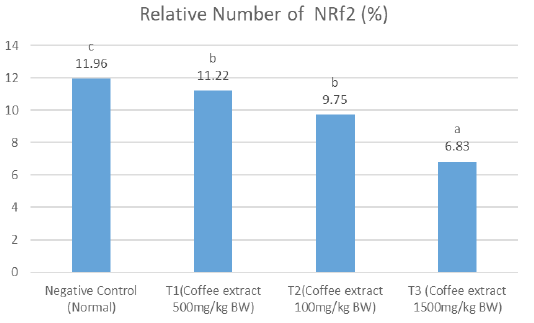
Figure 2: A significant differences notation (a, b c and d) showed between treatments for the relative level of Nrf2 (P <0.05). T1, T2 and T3 showed lower Nrf2 levels than negative controls. This shows that robusta green coffee extract can reduce levels of Nrf2.
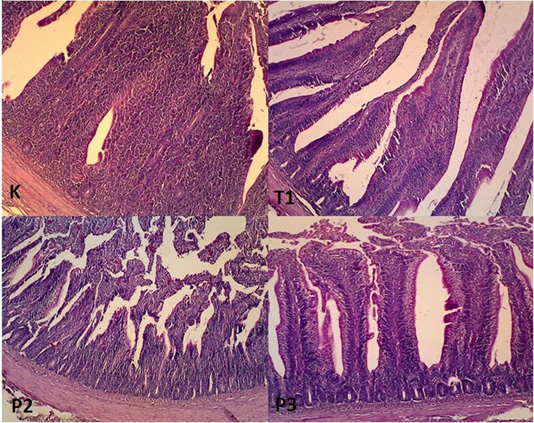
Figure 3: Histophatology of duodenum organ in magnification 400x. K: Layers arrangement of epithelium columnar simplex, no erosion found (Magnification 400x). T1: Layers arrangement of simple columnar epithelium, found a slight erosion (Magnification 400x). P2: Layers arrangement of epithelium simple columnar, found a slight erosion (Magnification 400x). P3: Layers arrangement of epithelium simple columnar, found an erosion (Magnification 400x).
Duodenum histopathology
Histopathological examination of duodenum organ showed that the epithelial arrangement in negative control showed layers arrangement of epithelium columnar simplex, no erosion found Figure 3. The composition of the epithelium in T1 found layers arrangement of simple columnar, found a slight erosion (Magnification 400x). In T2 the epithelial arrangement consists of a simple columnar and slight erosion. In T3 simple columnar simplex, found an erosion.
Table 1: Effect of coffee extracts on the height of duodenum villi.
| Treatment | Vili height (µm) |
| Negative control |
98.40±2.67d |
| T1 |
84.26±3.09c |
| T2 |
70.62±1.09b |
| T3 |
61,04±1.19a |
Note: Differences in notations a, b, c and d indicate a significant difference (P <0.05) between groups. Negative control, T1 (500 mg / kg BW), T2 (1000mg / kg BW) and T3 (1500 / kg BW).
Effect of coffee extract on the height of duodenal villi
The action of the small intestine correlates with the formation of intestinal villi which can be influenced by several factors such as the type of feed ingredients, feed chemicals, and feed additives as well as possible to disruption growth of the small intestine villi which can be illustrated through histopathological features. One parameter that can be observed is the height of villi. Villi height was measured from the tip (lamina propira) from villi to the base (villus crypt junction) (Nasir et al., 2013).
The results of the statistical analysis height villi of duodenum given coffee extract showed significantly higher negative control and treatment 1 (500 mg / kg body weight) the treatment that was most close to the negative control value, this showed that treatment 1 (500 mg / kg body weight) is more effective and leads to lower villi height reduction followed by treatment 2 (1000 / mg kg body weight) and treatment 3 (1500 / kg body weight) (Table 1).
DISCUSSIONS
Inflammation is a complex reaction in the vascularized connective tissue due to exogenous or endogenous stimuli. In the simplest sense, inflammation is a protective response aimed at eliminating the initial cause of cell injury and removing cells and necrotic tissue caused by cell damage (Robbins, 2004). Causes of inflammation include microorganisms, mechanical trauma, chemicals, and physical effects. The ultimate goal of the inflammatory response is to attract plasma proteins and phagocytes to the site of injury or invasion to isolate destroys, or activate an agent that enters, cleans debris and prepares tissue for the healing process (Khanna et al., 2014).
The mechanism of antioxidants can occur directly or indirectly. It can directly occur by donating hydrogen ions so that it can neutralize the toxic effects of free radicals, while the mechanism is indirectly by increasing the expression of endogenous anti-oxidant genes through several mechanisms. One mechanism of increasing the expression of anti-oxidant genes is through the activation of nuclear factor erythroid2 related factor 2 (Nrf2) which is a gene that plays a role in the synthesis of endogenous antioxidant enzymes (Kamilatussainah, 2015).
Nrf2 plays an important role in the regulation of cellular defense against endogenous and exogenous stress induced (Kenstler et al., 2007). Nrf2 induces HO to increasing mrna and protein correction. The metabolism of ho-1 has a significant anti-inflammatory effect with mediation by Nrf2 would helping to overcome the nf-kb signal. It can help reduce inflammation of the intestinal mucosa. NF-κB is a complex protein that having responsible for DNA transcription which is founded in almost all types of animals and its involved in all processes such as apoptosis, inflammation, immune response, cell growth and development (Syed et al., 2016).
The results of the consecutively relative number HO-1 level showed value of negative control (29.68%), T1 (19.25%), T2 (15.95%), and T3 (14.43 %).
The results of the relative calculation of Nrf2 level showed value of negative control 11.96%, T1 (500mg / kg BW of coffee extract) showed a value of 11.22%, T2 (1000mg / kg BW of coffee extract) showed 9.75%, and T3 (1500mg / kg BW of coffee extract) coffee extract) shows a value of 6.83%. The results of Nrf2 and HO-1 both decreased in each treatment from T1 to T3. According to Priftis et al. (2018), the higher dose of coffee extract given can cause a decrease in Nrf2 levels.
Coffee has the highest polyphenol concentration among the analyzed drinks. The main polyphenol in coffee is Chlorogenic acid (CGA). Chlorogenic acid (CGA) is an ester formed from cinnamic acid and quinic acid and also known as 5-O-caffeoylquinic acid (5-CQA) (IUPAC numbering) or 3-CQA (pre-IUPAC numbering). The most common tipe form of CGA is 5-caffeoylquinic acid (5-CQA). Accumulated evidence has shown that CGA exhibits many biological properties, including antibacterial, antioxidant, and anti-carcinogenic activities, especially hypoglycemic and hypolipidemic effects. CGA has recently been claimed to modulate glucose and lipid metabolism in vivo under conditions of healthy and genetic metabolic disorders. CGA does promote a significant reduction in plasma glucose peaks in oral glucose tolerance tests, most likely by weakening intestinal glucose absorption, suggesting a possible role for CGA as a glycemic index reduction agent. CGA, caffeine, and other polyphenol compounds in green coffee bean extract (GCBE) react to suppress weight gain and accumulation of visceral fat in mice. The authors report that CGA might be effective against weight gain and fat accumulation (Meng et al., 2013).
The results of measurement of height villi showed decrease along dose of coffee extract. This is contrary to Chen et al. (2018) which states that villi height, crypt depth and ratio of villi height to crypt depth are considered as criteria to reflect the morphology of intestinal mucosa and absorption capacities small intestine. Thus, an increase in villi height, the depth of the villi / crypt ratio corresponds to an increase in digestion and nutrient absorption. CGA supplementation can increase villi / crypt ratio and villi height in jejunum and ileum and decrease crypt depth in studies using rats.
The high ingredients of active CGA can reduce NFR2. At high doses, toxicity can occur due to the shifting of antioxidants into prooxidants from polyphenols (Priftis et al., 2018). The relative cell counts of Nrf2 and HO-1 are optimal in Treatment 1, close to negative control or healthy chicken treatment. An increase of relative amount is thought due to the presence of polyphenol compounds. Polyphenols are the result of secondary metabolism from plants which play an important role in cellular function and are found in coffee. Depending on its concentration, the activity of polyphenol can shift from antioxidant to prooxidant. Polyphenol has potential to cause depression in Nrf2, which can be seen in the treatment of T2 and T3 (Priftis et al., 2018).
The decrease of Nrf2 in this study correlates with decrease of HO-1. The effect of Nrf2 and HO-1 can be observed through histopathological features of the duodenum organ. Histopathology of duodenum organ devective suspected to be due to CGA which is prooxidant. The redox cycle of CGA produces oxygen compounds that are catalyzed by transition metals (Cu and Fe) to form reactive oxygen species that can damage macromolecules such as DNA and lipids (Liang and David, 2016). Nrf2 in protecting the intestinal mucosa by keap 1 induces Nrf2 translocation from the cytoplasm to the nucleus and activates the expression of target genes (Wen et al., 2019). It makes inflammation in the duodenum can be reduced. Nrf2 activation can inhibit the inflammatory pathway and reduce cytokine production. The disseminated keap 1 suppresses ubiquitination and IKB degradation thereby inhibiting NF-KB activation.
Exogenous and endogenous stimuli capable to cause injury in cells and cause inflammatory reactions in the form of complex reactions in tissues that have vascularity. Depends on the depth injury such as in the intestine, the erosion reaction in mucosal erosion appear by loss of part of the thickness of the mucosa and mucosal ulceration appear by loss of the entire thickness of the mucosa and sometimes even deeper defects to reach the propria muscularis (Underwood, 1999).
Caffeine is an alkaloid compound in the form of white crystals. Caffeine is an ingredient in coffee beans that can inhibit bacterial growth, where Robusta coffee has a content of 1.6% -2.4% (Widyotomo and Mulato, 2007). The ability of alkaloids is strongly influenced by the biological activeness of these compounds, which is caused by the presence of a nitrogenous base group. This base group will react with amino acid compounds when alkaloid compound contact with bacteria that make up with cell wall and bacterial DNA which is the main constituent of the cell nucleus and center of all cell activity. This reaction occurs in chemically a base compound which react with an acid compound, in this case amino acids. The result of this reaction cause alteration of the structure and composition of amino acids and it can occur by most reacted of base alkaloid groups compounds with amino acids. Alteration in the composition of these amino acids will obviously changing the arrangement of DNA chains in the nucleus of cells which originally had a pair of acids and bases in pairs. The alteration in the composition of amino acid chains in DNA will cause changes in genetic balance that will makes damaged bacterial DNA. Because DNA is the main component of a cell’s nucleus, damage of the DNA will cause the damage of bacterial cell’s nucleus. DNA damage in the bacterial cell nucleus will also promote lysis of the nucleus of that bacterial cell. Lysis of the nucleus bacterial cell can cause cell damage to bacteria by interference of center cell activity. Over time it will make bacterial cells unable to metabolize and undergo lysis. Then bacteria will become inactive and destroyed (Gunawan, 2009). It is assumed that the way caffeine works to inhibit bacterial growth is the same as the caffeine in Robusta coffee bean extract.
The caffeine at high doses can cause damage to the intestinal mucous membranes. Intestinal epithelium acts as a natural barrier against pathogenic bacteria and toxic substances present in the intestinal lumen. Stressors, pathogens, and chemicals can cause disruption in normal micro flora that can change the permeability of the intestinal epithelium, facilitate the infection of pathogens and harmful substances, modify metabolism, the ability to digest and absorb nutrients, and can lead to inflammatory processes in the intestinal mucosa (Pelicano et al., 2005).
Other components besides caffeine that found in Robusta coffee beans which are also reported to have antibacterial activity such as phenol compounds, trigonelline and chlorogenic acids (Fardiaz, 1995). Phenol compounds are flavonoids found in coffee beans. Biological activity of flavonoid compounds by spoil bacterial cell walls, through differences in polarity between the constituent DNA lipids with alcohol groups in flavonoid compounds its make cell walls disturbed and these compounds can enter the bacterial cell nucleus. The mechanism of biological activity by these flavonoid compounds is different from those carried out by alkaloid compounds, in which flavonoid compounds damage bacterial cells. Whereas the alkaloid compounds utilize the reactive nature of alkaline groups on alkaloid compounds to react with amino acid groups on bacterial cells (Gunawan, 2009).
CONCLUSION
Based on the results of research it can be concluded that the Robusta Lampung coffee extract (coffea canephora) that 500 mg/kg dose of coffee extract (T1) has potential anti-inflammatory and anti-oxidant effect in chicken is erroneous because all treatments showed significantly lower amounts of Nrf2 and HO-1 than the negative control.
ACKNOWLEDGMENT
Thank you to the 2018 College of Higher Education Research funders, the Ministry of Research and Technology and Universitas Brawijaya.
AUTHORS CONTRIBUTION
DR received research funding, DR and LER made Research concept and Design; LER, DQS data analysis; IAA, DP, FSP and DQS conducted research. All team members wrote and revised article.
Conflict of interest
There is no conflict of interest between the research teams
REFERENCES






