Advances in Animal and Veterinary Sciences
Research Article
Biochemical Properties and Antibiotic Sensitivity of Isolated E. Coli from Neklinovsky District of the Rostov Region, Russia
Alexander Alekseevich Shevchenko*, Oleg Urievich Chernykh, Anastasia Ruslanovna Litvinova, Andrey Georgievich Koshchaev, Natalya Evgenievna Gorkovenko
Kuban State Agrarian University, Krasnodar, Kalinina Street, 13, 350044, Russia.
Abstract | The objective of the present work is to study the biochemical properties of E. coli isolates and to determine their antibiotic sensitivity with regard to antibacterial drugs used in the agricultural field. The work was carried out in two farms of the Neklinovsky District of the Rostov Region in the period from 2015 to 2018. To determine the formation of indole, culture was plated in Hottinger Broth, five samples (#1, #2, #131, #139 and #1371) out of all sixteen collected samples revealed red layer on the surface of the culture fluid, indicating a positive response. A ring that appeared the next day confirmed the presence of indole. Meanwhile, to determine the ability to utilize citrate, cultures were plated on the surface of Simmons medium, and the same five samples (#1, #2, #131, #139 and #1371) gave green color for the medium changed to blue, indicating positivity. All the tested samples except sample #1371 fermented glucose, sucrose, lactose, and mannitol, which is typical for Escherichia coli. Likewise, further characterization to determine the formation of hydrogen sulfide, cultures were plated on three-sugar agar, and one sample out the previous five samples make blackening on the medium column, indicating the formation of hydrogen sulfide Thus, out of the sixteen studied cultures, sample # 1371 can be attributed to bacteria of the Pseudomonas aeruginosa species while the four cultures #1, 2, 131, and 139 belong to the E. coli + Citrobacter freundii bacteria group, however the remaining 11 cultures belong to the Escherichia coli bacteria group. Sensitivity for the antibiotic susceptibility of genus Escherichia to antibacterial drugs (meropenem, imipenem, pefloxacin, enrofloxacin, carbenicillin, cefotaxime, ceftriaxone, vancomycin, furadonin, fosfomycin, gentamicin, netilmicin, colistin, levofloxacin, tetracycline, laevomycetin, ciprofloxacin, chloramphenicol, norfloxacin, furagin, vancomycin, spectinomycin, doxycycline, and norfloxacin) was determined using standard disks. This study shows that in eight cultures of genus Escherichia were most sensitive to fosfomycin, enrofloxacin, imipenem, meropenem, levofloxacin, vancomycin, and gentamicin while nine cultures were sensitive to enrofloxacin, imipenem, meropenem, levofloxacin, vancomycin, gentamicin, cefotaxime, and norfloxacin. While conducting the research, the bacterial microflora was identified, which was the causative agent of infectious diseases. An antibiotic sensitivity had also been identified, which allowed for more effective treatment regimen for cattle.
Keywords | Escherichiosis, Antibiotics, Bacteriological study, Feces, Liquid media, Antimicrobial susceptibility
Received | August 01, 2020; Accepted | August 7, 2020; Published | August 28, 2020
*Correspondence | Alexander Alekseevich Shevchenko, Kuban State Agrarian University, Krasnodar, Kalinina Street, 13, 350044, Russia; Email: shevhenko_aa@rambler.ru
Citation | Shevchenko AA, Chernykh OY, Litvinova AR, Koshchaev AG, Gorkovenko NE (2020). Biochemical properties and antibiotic sensitivity of isolated E. Coli from Neklinovsky District of the Rostov region, Russia. Adv. Anim. Vet. Sci. 8(s3): 33-37.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s3.33.37
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Shevchenko et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Animal husbandry in Russia ranks of high priority in agriculture and faces the task to ensure a full-fledged supply of livestock products to the consumer, and raw materials to the industry (Toropyno et al., 2018). The importance of veterinary interventions is increasing against a backdrop of high concentration, in-depth specialization, and agro-industrial integration of animal husbandry, the creation of industrial complexes in which a large number of animals are concentrated in a limited area (Koshchaev et al., 2018; Troshin et al., 2018; Skvortsova et al., 2018; Semenov et al., 2019). So, the importance of veterinary workers, who must provide preventive measures to combat infectious diseases, increases significantly.
Infectious diseases of the gastrointestinal tract in young cattle cause significant economic damage to farms, due to the loss of cattle, growth retardation, reduction in the gain of live weight, culling of sick animals, and high costs of carrying out measures for their prevention and elimination (Bakharev et al., 2018; Tuzov et al., 2018; Anisimova et al., 2018; Kiselev et al., 2019; Kulikova et al., 2019; Koshchaev et al., 2019). Currently, among of these infectious diseases of young cattle, a special place is occupied by gastrointestinal diseases of bacterial etiology. The leading role in the nosological profile of these diseases is played by pathogenic strains of Escherichia coli, which cause gastrointestinal Escherichiosis, which consider a main problem in farms not only in Russia but even worldwide (Bogomolov, 2000; Sukhinin et al., 2015; Toropyno et al., 2018; Urban, 1970; Kaper et al., 2004; Koshchaev et al., 2017, 2018; Saleeva et al., 2018; Anisimova et al., 2019; Tyurin et al., 2019).
For prevention and control of Escherichiosis, preference is given to vaccination and antibiotic therapy, while antibiotics are not always effective due to low sensitivity to certain strains of E. coli because bacteria quickly become resistant to antibiotics (Pozdeev, 2001; Shevchenko et al., 2009; Shevchenko and Toropyno, 2017; Matiuzzi et al., 2008; Serdyuchenko et al., 2018; Garkovenko et al., 2018; Koshchaev et al., 2018; Kryukov et al., 2018; Svistunov et al., 2019; Nikitin et al., 2019).
The use of new combination of probiotic bacteria is one of the current trends in the prevention of gastrointestinal infections in calves (Matiuzzi et al., 2008; Shevchenko and Dvadnenko, 2012; Chernykh et al., 2017; Hagberg et al., 1981; Zverzhanovskiy et al., 2017; Koshchaev et al., 2018; Koba et al., 2018; Ratoshny et al., 2018; Troshin et al., 2018; Yuldashbaev et al., 2019). At the moment, when selecting therapy and prevention measures against the disease, it is advisable to monitor the sensitivity of pathogenic and opportunistic pathogens to antibiotics. While conducting the research, the bacterial microflora was identified, which was the causative agent of infectious diseases. An antibiotic sensitivity had also been identified, which allowed for more effective treatment regimen for cattle.
MATERIALS AND METHODS
In the course of conducting the present work, feces from calves between 1-8 days of age were collected and examined for Escherichia. Laboratory diagnostics on Escherichiosis of calves was carried out in two farms, namely, “50 Years of October” and “Rus” of the Neklinovsky District of the Rostov Region according to methodical instructions on bacteriological diagnostics of colibacteriosis (escherichiosis) of animals (Toropyno et al., 2018). Feces were sampled by means of sterile cotton-gauze tampons directly from a rectum. The following nutrient media were used to identify escherichia: Meat-peptone broth and agar, Endo agar, Minca Agar, Simmons nutrient agar, Giss media with sugars, urea media, iron sulfate media, Levin medium, and Hottinger broth. Diagnostic Givental-Vedmina agar (AGV) and E. coli culture propagated for 18 hours according to the turbidity standard to determine the sensitivity. Next, 1 ml of the culture suspension was poured on the surface of the medium and evenly distributed over the entire surface, the slightly opened dishes were dried at room temperature for 10-15 minutes and discs with various antibiotics were laid out using sterile tweezers on the surface of the seeded medium at the same distance from each other and at a distance of 2 cm from the edge of the dish. The results were taken 24 hours after the thermostat and the growth retardation zone was measured by means of a ruler with respect to antibiotics.
RESULTS AND DISCUSSION
During the bacteriological test, sixteen cultures were subjected for biochemical tests. The bacterial cultures were plated on a Giss media with glucose, sucrose, lactose, mannitol, urea medium, citrate Simons agar, and 2 tubes of beef-extract broth (BEB) (tests for indole and hydrogen sulfide) (Table 1).
To determine the formation of indole, culture was placed into a test-tube with Hottinger broth. Then 1 cm3 of Kovacs reagent was added to 5 cm3 of the propagated bacterial culture. Five samples (#1, #2, #131, #139 and #1371) out the sixteen tested samples showed red layer on the surface of the culture fluid, indicating a positive response and a ring that appeared next day confirmed the presence of indole.
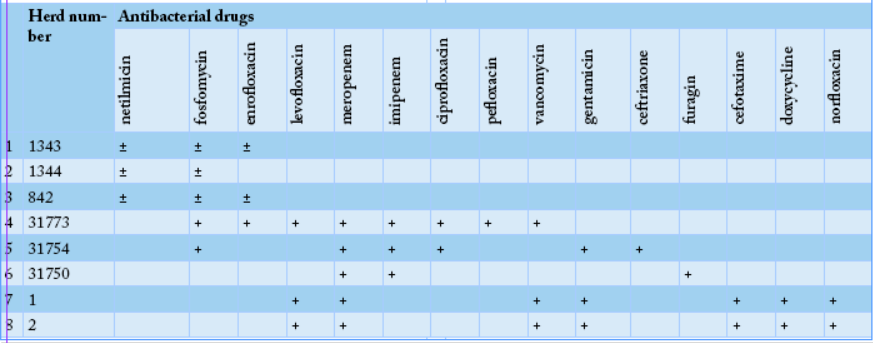
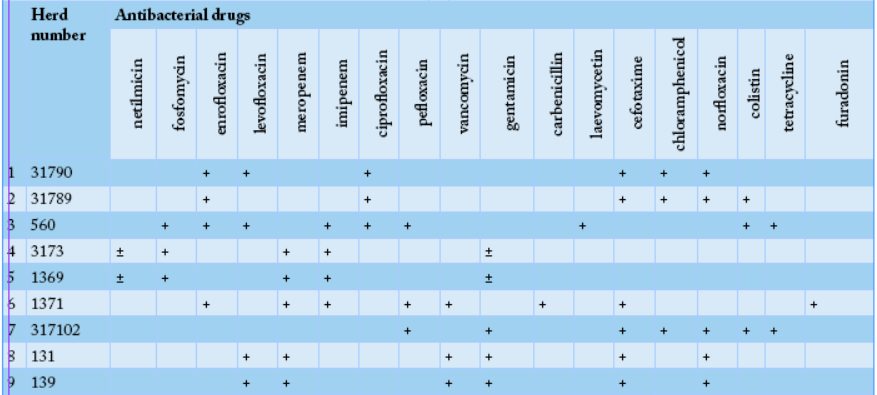
To determine the ability to utilize citrate, bacterial cultures were plated on the surface of the Simmons medium, samples #1371, #1, #2, #131, #139 and #1371 showed green color of the medium changed to blue, indicating positivity of E. coli. All the tested samples except sample #1371 fermented glucose, sucrose, lactose, and mannitol, which was typical for E. coli. To determine the formation of hydrogen sulfide, cultures were plated on three-sugar agar, and the test-tubes were incubated at 36oC for 24 hours. Culture of sample #1371, it noticed blackening on the medium column, indicating the formation of hydrogen sulfide. In 11 samples except of the samples #1, 2, 131, 139, and 1371, hydrogen sulfide was not produced. Thus, out of the sixteen studied E. coli cultures, sample # 1371 did not ferment glucose and lactose with the formation of acid and gas, had shown growth on Simons agar and did not form indole and hydrogen sulfide, which allowed classifying it to Pseudomonas aeruginosa species. Moreover, samples # 1, 2, 131, and 139, cultures did not break down urea, but break down lactose, fermented glucose to form gas, produced hydrogen sulfide, and did not form indole, suggesting that it was beloning to the E. coli + Citrobacter freundii group. Likewise, the remaining eleven samples were able to ferment glucose and lactose with the formation of acid and gas, did not show growth on Simons agar, did not utilize citrate-ammonium salts, did not break down urea, but formed indole, and did not form hydrogen sulfide which indicate that they were belonging to thethe E. coli group. Sensitivity of Escherichiosis cultures to the antibacterial drugs; meropenem, imipenem, pefloxacin, enrofloxacin, carbenicillin, cefotaxime, ceftriaxone, vancomycin, furadonin, fosfomycin, gentamicin, netilmicin, colistin, levofloxacin, tetracycline, laevomycetin, ciprofloxacin, chloramphenicol, norfloxacin, furagin, vancomycin, spectinomycin, doxycycline, and norfloxacin was tested using standard discs. The sensitivity of escherichiosis cultures to antibacterial drugs in different farms was varying as shown in Tables 2 and 3.
Table 1: Study of biochemical properties isolated from E. Coli feces cultures.
| Farms | Code number of calf | Media | ||||||||
|
Giss media
|
Simons agar | Urea | with Fe sulfate |
BEB (for indole) |
slant MPA | |||||
| glucose | sucrose | sucrose | mannitol | |||||||
| 50 Years of October | 31790 | + | + | + | + | - | - | - | + | + |
| 31789 | + | + | + | + | - | - | - | + | + | |
| 560 | + | + | + | + | - | - | - | + | + | |
| 3173 | + | + | + | + | - | - | - | + | + | |
| 1369 | + | + | + | + | - | - | - | + | + | |
| 1371 | - | - | - | - | + | + | + | - | - | |
| 1 | + | +- | +- | + | + | +- | + | - | _ | |
| 2 | + | +- | +- | + | + | +- | + | - | _ | |
| Rus | 1343 | + | + | + | + | - | - | - | + | + |
| 1344 | + | + | + | + | - | - | - | + | + | |
| 842 | + | + | + | + | - | - | - | + | + | |
| 31773 | + | + | + | + | - | - | - | + | + | |
| 31754 | + | + | + | + | - | - | - | + | + | |
| 31750 | + | + | + | + | - | - | - | + | + | |
| 131 | + | +- | +- | + | + | +- | + | - | _ | |
| 139 | + | +- | +- | + | + | +- | + | - | _ | |
Thus, analyzing the data of eight cultures presented in Table 2, one can state that in the first farm genus Escherichia was the most sensitive to antibiotics, such as fosfomycin, enrofloxacin, imipenem, meropenem, levofloxacin, vancomycin, and gentamicin.
Our results revealed that bacteriological study of feces from a calf, sample #1371 allowed the isolation of Pseudomonas aeruginosa, while cultures of samples # 1, 2, 131, and 139 were belonging to E. coli + Citrobacter freundii group. Eleven cultures were attributed to bacteria of the genus Escherichia. In the “50 Years of October” farm, eight bacterial isolates of genus Escherichia have shown the highest antibiotic sensitivity to fosfomycin, enrofloxacin, imipenem, meropenem, delafloxacin, and gentamicin (Table 2). While, in the “Rus” farm, nine bacterial culture isolates of the genus Escherichia have shown the highest sensitivity to enrofloxacin, imipenem, meropenem, levofloxacin, vancomycin, gentamicin, cefotaxime, and norfloxacin (Table 3).
After analyzing the data of Table 3, in the second farm, out of the nine cultures, genus Escherichia was the most sensitive to enrofloxacin, imipenem, meropenem, levofloxacin, vancomycin, gentamicin, cefotaxime, and norfloxacin.
CONCLUSION
While studying the bacterial microflora of cattle, the Pseudomonas aeruginosa and Citrobacter freundii pathogens were identified, which were a consequence of the disease in cattle, causing damage to production. The revealed sensitivity of bacteria to antibiotics made it possible to draw up more effective treatment regimens for young cattle.
Authors Contribution
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES