Advances in Animal and Veterinary Sciences
Research Article
Commingle Consumption of Monosodium Glutamate and Aspartame and Potential Reproductive System Affection of Female Albino Rats: Involvement of VASA Gene Expression and Oxidative Stress
Heba El-Sayed Mostafa1,Islam Mohamed Magdi Ammar2, Dalia Abdallah El-Shafei3, Amal Nooredeen Ahmed Allithy4, Nassar Ayoub Abdellatif5, Eman Ahmed Alaa El-Din1*
1Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 2Department of Obstetrics and Gynecology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 3Department of Community, Environmental & Occupational Medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 4Departments of Pathology, Faculty of Medicine, Sohag University, Egypt; 5Department of Anatomy, Faculty of Medicine, Sohag University, Egypt.
Abstract | Monosodium glutamate (MSG) and Aspartame (ASP) are the most commonly used food additives. Despite their wide-spread, their safety was examined and fears of toxic effects were recently expressed. So, this study aimed to assess the separate and commingle effects of monosodium glutamate and aspartame on some reproduction function of adult female albino rats. A total of Thirty-two female rats were divided randomly into 4 equal groups (8 rats/ group). Group I: Control rats were fed on a standard diet. Group II:(MSG) each rat received MSG (400mg/Kg. body weight). Group III: (ASP) each rat administrated ASP (40 mg/Kg. body weight). Group IV: (MSG + ASP) each rat received MSG and ASP at the same previous dosage regimen for the same duration. All the treatment was orally gavaged daily for 30 days. Either MSG or ASP individually or mixed had significant deleterious effects on the ovarian functions of the rats in the order MSG then ASP. Aspartame alone was unable to alter the estrogen level or VASA gene expression and caused no considerable histopathological changes of the ovaries, while the combination of (MSG+ASP) decreased significantly the levels of estrogen and gene expression and produced destructive histopathological changes in the pituitary and ovarian tissues leading to reproductive dysfunction. This could be explained by MSG and ASP-induced pronounced ovarian redox imbalance evidenced via generating lipid peroxidation with diminishing reduced glutathione. More researches are mandatory to assess the adjuvant impact of monosodium glutamate and aspartame on female reproductive function and antioxidant defense systems.
Keywords | Flavoring agents, Artificial sweetener, Ovary, Estrogen, Pituitary
Received | January 09, 2021; Accepted | January 28, 2021; Published | March 30, 2021
*Correspondence | Eman Ahmed Alaa El-Din, Department of Forensic Medicine and Clinical Toxicology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; Email: Eman_alaa77@yahoo.com
Citation | Mostafa HS, Ammar IMM, El-Shafei DA, Allithy NA, Abdellatif NA, Alaa El-Din AA (2021). Commingle consumption of monosodium glutamate and aspartame and potential reproductive system affection of female Albino rats: involvement of VASA gene expression and oxidative stress. Adv. Anim. Vet. Sci. 9(5): 700-708.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.700.708
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Alaa El-Din et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Evaluating the safe consumption of food additives is commonly accepted after testing their toxicities through animal studies as there is scarce data from human studies. The main aim of animal studies is to evaluate the additives’ potential risks for human toxicity and to establish the conditions for safe use (Lin et al., 1992).
Monosodium glutamate is a crystalline powder that is composed mainly of glutamic acid and to some extent of sodium, and water. Glutamate is a non-essential amino acid that is the responsible part of MSG accounting for adversely affects humans (Abbasi et al., 2006). Monosodium glutamate is considered one of the commonly used flavors all over the world. It exists in numerous assortments of different foods (Rim, 2017; Bojanić et al., 2009). Unfortunately, various researches revealed that MSG has various toxic effects (Abdollahzadeh et al., 2017). However, the majority of these studies have been still focusing on its neurotoxic potentials (Afolabi et al., 2020).In defiance of all the debate regarding the toxicity of MSG; it is also used in huge doses in treated foods. Accordingly, fears have increased because of the toxic effects of MSG, with minimal research about the cytotoxic damage of ovaries with MSG exposure as its effects on the reproductive system were out of the scope of most previous studies (Husarova and Ostatnikova, 2013).
Aspartame (ASP), is a synthetic sugar-substitute that is consumed in various products like drinks and bubble gum. It is well absorbed in the digestive tract and converted into phenylalanine, aspartic acid, and methanol. Accordingly, extensive use of ASP increases the blood levels of these metabolites. Formaldehyde is converted to formic acid which is the end metabolic product of methanol. Format causes inhibition of cytochrome oxidase, ending with the formation of free radical scavengers like superoxide, peroxide, and hydroxyl that may increase oxidative stress and lead to DNA damage (Ozturan et al., 2017; Al-Eisa et al., 2018).
Although numerous studies about ASP support its safety, there is still a lack of evidence about its long-term and high-dose consumptions (Gardner, 2014). Various studies documented its hazardous effects such as ASP-related neurological disturbances, mood affections, digestive symptoms, and hypersensitivity syndromes. Also, an irregular menstruation cycle has been documented (Coulombe and Sharma, 1986). Moreover, the ingestion of ASP by pregnant and lactating animals could affect the descendants’ structural and functional maturity (Abu-Taweel et al., 2014).
The female reproductive system is extremely susceptible to the most exposed chemicals. Presently, the etiologies of ovarian dysfunctions remain largely unknown. VASA gene is a genetic stamp that could be useful in the visualization and identification of primordial germ cells (PGCs).They are progenitors for germ cell line accountable for transmitting genes to the progeny (Wang et al., 2012; Wang et al., 2014).
Various previous animal studies on MSG and ASP have documented a variety of hazardous effects for each one separately. However, the impacts of MSG and ASP separate consumption on female reproductive organs were recognized in limited studies moreover, their amalgamate effects still unclear. Therefore, our study aimed to explore some aspects of the potential reproductive toxicity of MSG and ASP single and combined consumption on the ovarian function and pituitary gland of adult albino rats.
Materials and Methods
Chemicals
Monosodium glutamate and Aspartame: obtained from Sigma Company in Cairo city, Egypt.
Animals
Thirty-two female rats, 2 to 3 months and weighing 100-150g, were group-housed under controlled temperature and light conditions at Animal House of the Faculty of Medicine, Zagazig University. Rats were allowed standard laboratory rodent chow and water ad libitum and kept for an adaptation period of 2 weeks. The study followed the recommendations of Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council (2011) and in line with guiding principles of Institutional Animal Care and Use Committee (IACUC), Faculty of Medicine, Zagazig University, Egypt.
Experimental design
After rats have been acclimatized, they were randomly separated into 4 groups (each group includes 8 rats). All the treatments were dissolved in tap water and orally gavaged for a period of 30 days.
Control group (I): Rats were given plain tap water and fed on a standard diet.
Group II: rats were treated with MSG (400 mg/Kg. bwt.), dosage was matched with Takasaki (1978); Sant’Diniz et al. (2005); Waer and Edress (2006). LD50 of orally administered MSG in rats and mice is 15.000 -18.000 mg/kg bwt.(Walker and Lupien, 2000; Oladipo et al.,2015).
Group III: each rat received ASP at a daily dose of 40 mg/Kg bwt. according to European Food Safety Authority (EFSA)(2006); Choudhary and Devi (2016).
Group IV (MSG+ ASP-treated group): rats were treated with both MSG (400mg/Kg. bwt.) plus ASP (40 mg/Kg bwt.) for 30 days.
At the end of the thirty days, the animals were weighed and then the rats of all groups were anesthetized with sodium pentobarbital (Tsubokura et al., 2016), to lessen pain and stress and blood was withdrawn then centrifuged to obtain sera. Samples were used for performing the biochemical studies; then rats were then euthanized for tissue collection (ovaries and pituitary glands) (Figure 1).
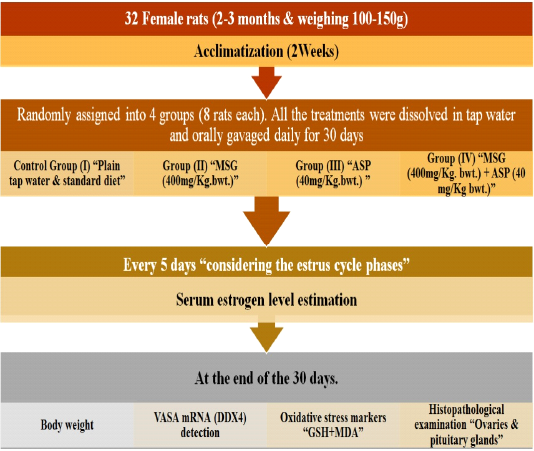
Figure 1: Experimental plan
Methods
Bodyweight
By the end of the experiment, the body weights of the animals were estimated.
Estimation of serum estrogen level
Every 5 days considering the estrus cycle’ phases, blood samples were withdrawn from each rat. Enzyme-linked immunosorbent assay (ELISA kit) was used to measure serum estrogen levels according to the method of Faccio et al. (2013).
VASA mRNA (DDX4) detection via (qRT-PCR)
Total RNA from ovaries was isolated from 250 mg tissues using the Qiagen R Neasy Midi Kit (Qiagen, Valencia, CA, No.75144) following the manufacturer’s instructions. The whole ribonucleic acid concentration was determined and 5 μg total RNA from the tissue was treated with RNAse-free DNAse (2 units/μg RNA, Promega, Madison, WI, No. M6101) at 37°C for 60 min to eliminate any genomic DNA that might contaminate the sample. Next, 200 ng treated RNA has been reversely transcribed by two-hundred units SuperScript III Reverse Transcriptase (Life Technologies, No. 18080-044) in 20 μl final volume at 50°C for 60 min in the presence of 40 units RNase Inhibitor (Life Technologies, No. 10777-019) using Oligo(dT)20 Primers (Life Technologies, No. 18418-020). For no reverse transcriptase (RT) controls, the enzyme was replaced with double-distilled H2O for each sample. For SYBR Green quantitative polymerase chain reaction (PCR), 0.5 μlc DNA was used as a template in a final volume of 10 μl per reaction. Polymerase chain reaction reactions were performed in triplicate for each tissue sample. EGFP primers were forward, 5΄-GAACGGCATCAAGGTGAACT-3΄, and reverse 5΄-TGCTCAGGTAGTGGTTGTCG-3΄. As an internal control, rat-specific ß-actin was amplified from each sample with primers forward, 5΄-CCCTGTGCTGCTCACCGAGG-3΄, and reverse 5΄-GGCTACGTACATGGCTGGGGTG-3΄. Real-time PCR was executed on the 7900 HT Fast Real-Time PCR System (Applied Biosystems from Life Technologies) with SDS 2.4 software. Relative EGFP expression for each tissue was estimated using the delta-delta Ct (ΔΔCt) method (Gassei et al., 2017).
Oxidative stress markers
Ovarian tissues homogenate was used for measuring the following oxidative parameters:
Reduced glutathione (GSH): as an enzymatic anti-oxidant in ovarian tissues. It was estimated following the method of Melekoglu et al. (2018). Malondialdehyde as an end product of lipid peroxidation in ovarian tissues. So, the tissue LPO level was considered by measuring the malondialdehyde levels (MDA). The method of Mahran et al. (2015) was followed to measure MDA.
Histopathological examination
The ovaries and pituitary were immediately dissected out and the specimens were fixed with 10 % neutral formalin for more than 12 hours, dehydrated, set in paraffin, sections were cut at 5 μm, then preparing slides and staining by hematoxylin and eosin (H&E) stain (Bancroft and Layton, 2013).
Statistical analysis
Results were summarized by mean ± Standard deviation (X±SD), then analyzed by computer using the SPSS program (Statistical Package for Social Science) version 16.0 (IBM, 2010). One-way analysis of variance test (ANOVA) used to determine a significant statistical difference, followed by the LSD test for multiple comparisons between different groups. The threshold of significance is fixed at 5% level (P-value) and highly significant at p-value <0.01 and <0.001.
Results
The measured variables for the control group were within the normal range.
Bodyweight
The bodyweight of both MSG (II) & ASP (III) treated groups was significantly P < 0.05 increased when compared with group (I) and a highly noteworthy P < 0.001 increase in MSG+ASP co-treated group (IV) (Table-1).
Table 1: Effect of MSG and ASP individually and in combination on body weight (gm) in adult female Albino rats
| Control group(I) | MSG-treated group(II) | ASP-treated group(III) | MSG+ ASP treated group(IV) | F | P |
LSD |
|
| Body weight (gm) | 187.17±11.04 | 230.54±13.8 | 220.25±24.55 | 255.36±20.09 | 19.373 | 0.000 |
<0.05a.b,c,e,f>0.05d |
a Group I versus group II, b Group I versus group III, c Group I versus group IV, d Group II versus group III, e Group II versus group IV, f Group III versus group IV
Values are expressed as mean ±standard deviation (SD) of n = 8 animals; *Significant difference (P < 0.05); **Highly significant difference (P < 0.001).
Table 2: Effect of MSG and ASP individually and in combination on serum estrogen levels (pg/mL) in adult Albino rats
| Serum estrogen (pg/mL) | Control group(I) | MSG-treated group(II) | ASP-treated group(III) | MSG+ ASP treated group(IV) | P* |
| P1 | 81.87±12.64 | 79.72±15.92 | 81.23±13.67 | 77.45±12.67 | 0.869 |
| P2 | 80.87±11.51 | 76.83±9.47 | 77.66±12.32 |
67.07±13.56a |
0.049 |
| P3 | 81.39±13.61 |
63.21±19.82a |
77.42±12.47 |
61.23±11.54b |
0.002 |
| P4 | 80.97±12.09 |
61.25±9.74a |
78.31±14.02 |
58.23±9.64b |
0.000 |
| P5 | 79.56±14.52 |
60.13±11.57a |
80.65±12.31 |
57.23±10.55b |
0.000 |
| P6 | 80.72±13.51 |
57.23±12.32a |
80.42±13.08 |
54.23±9.32b |
0.000 |
Values are expressed as mean ± standard deviation (SD) of n = 8 animals/group
*ANOVA test
a: P<0.05, compared with Control group
b: P<0.01, compared with Control group
Table 3: Comparison of mean values of oxidative stress markers in ovarian tissues in the study groups:
| Control group(I) | MSG-treated group(II) | ASP-treated group(III) | MSG+ ASP treated group(IV) | F | P |
LSD |
|
|
Reduced glutathione (nmol/mL) |
56.42 ± 12.67 | 41.35±11.72 | 38.98±6.13 | 26.11±5.03 | 13.700 | 0.000 |
<0.05a.b,c,e,f >0.05d |
|
Malondialdehyde (nmol/L) |
2.21 ± 0.63 | 14.25±3.21 | 10.64±1.14 | 38.61 ± 6.27 | 152.254 | 0.000 |
<0.05a.b,c,e,f >0.05d |
Table 4: Comparison of mean values of qRT-PCR relative gene expression of VASA (ng/ml) in the ovarian tissues of the study groups:
| Control group(I) | MSG-treated group(II) | ASP-treated group(III) | MSG+ ASP treated group(IV) | F | P |
LSD |
|
| qRT-PCR relative gene expression of VASA (ng/ml) | 10.46 ± 1.85 | 8.32±2.03 | 9.78±2.61 | 4.75±0.74 | 13.925 | 0.000 |
<0.05c,e,f >0.05a,b,d |
Serum estrogen assay
There were no observed changes in estrogen levels for the first time (P1) on the 5th day of the study but, a significant depletion in estrogen levels in MSG+ ASP treated group (IV) at the second time (P2) after 10 days of the study. While, during the 3rd, 4th, 5th, and 6th time (P3, P4, P5, and P6). Results revealed a significantly diminished estrogen level in MSG treated rats and a high significantly P<0.01 decrease in animals co-treated with MSG+ ASP (IV) when comparing to control and ASP treated group. Besides, rats of ASP treated group (III) showed no significant difference. It was as well observed significantly depleted estrogen levels in MSG and combined MSG+ ASP treated groups which were positively correlated to the duration of the study (Table- 2).
Oxidative stress
Malondialdehyde showed significantly increased levels in groups that separately received MSG or ASP in comparison to group (I). While rats of MSG+ASP mixed treatment group (IV) displayed a significant increase P<0.01 when comparing to other groups (I & II& III). Results showed a significant decline in reduced glutathione concentrations of all groups that received single or concomitant treatment (Table-3).
Results of VASA mRNA (DDX4) expression
Gene expression of VASA displayed significant down-regulation in group (II) received Monosodium glutamate P<0.05 and in combined MSG+ASP treated group (IV) P<0.01, in contrast, to control and ASP-treated groups that showing insignificant gene expression (Table-4).
Histopathological results
Ovaries: In control group I, ovarian tissue showed usual structural arrangement, having two distinctive anatomical divisions; the outer cortex and the inner medulla: the outer layer including follicles (ova with follicular cell and an inner central layer contains large blood vessels, lymphatic, nerve surrounded by a connective tissue stroma. Cortical layer is lined by squamous or low columnar epithelium sheltered beneath by several developmental stages of Graffian follicles prior to mature ovum liberate. The developing follicle has a large cavity (antrum) contain liquor folliculi and coated internally with zona granulosa cells and externally theca lutein (Fig.2A).
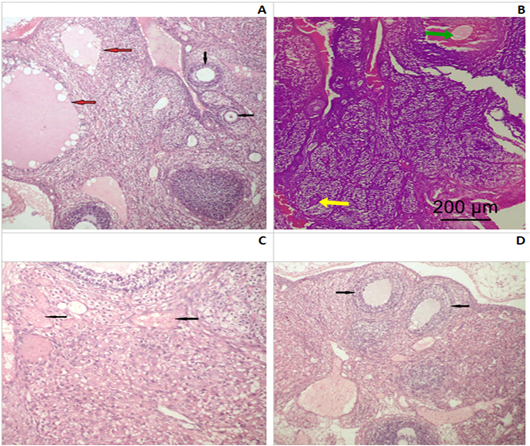
Figure 2: Photomicrograph of an ovarian section of adult Albino rats: (A) Control group I, the ovaries possessed normal histological architecture structure; well formed mature secondary follicles (red arrows) alternating with small primary follicles (black arrows). (B) Group II (SMG) showed less mature graffian follicles (yellow arrow) and congested blood vessels (green arrow). (C) Group IV (SMG +ASP) showed numerous markedly congested and dilated blood vessels with diffused hemorrhagic areas (black arrows). (D) Group IV (SMG +ASP) showed lower number of ovarian follicles with less maturity of the secondary follicles (black arrows) (H&E x200).
Monosodium glutamate treated group (II), ovarian tissue showed less mature graffian follicles and some congested blood vessels (Fig.2B). ASP treated group (III), had almost normal histological architecture structure and no obvious histopathological findings could be detected. While, MSG+ASP treated group (IV), showed less ovarian follicles with less maturity of the secondary follicles. In addition to previous findings, there were numerous markedly congested and dilated blood vessels with a diffused hemorrhagic area (Fig.2C and D).
Pituitary: Control group (I), exhibited a regular cytological structure with secretory cells and blood vessels (Fig. 3A). MSG treated group (II), showed a cystic formation and few fibrotic bands (Fig. 3B). ASP treated group (III), showed degenerated and necrotic areas with fragmented nuclei (Fig.3C). MSG+ ASP treated group (IV), showed a large fibrotic area involving most of the pituitary anterior lobe (Fig.3D).
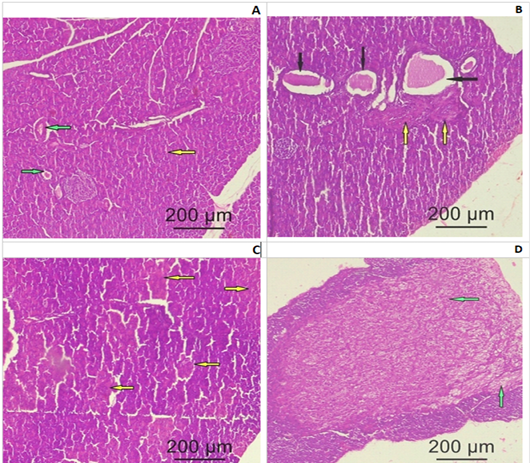
Figure 3: Photomicrograph of a pituitary gland section of adult female Albino rats: (A) Control group I showing normal histological structure of the secretory cells (yellow arrow) and blood vessels (green arrow) (B) Group II (SMG) show cystic formation (black arrows) and few fibrotic bands (yellow bands). (C) Group III (ASP) showing degenerated and necrotic areas with fragmented nuclei (yellow arrows). (D) Group IV (SMG +ASP) showing large fibrotic area (green arrow) involving most of the pituitary anterior lobe (yellow arrows) (H&E x200).
Discussion
One of the problematic issues today in many developed countries is female infertility because the female reproductive system is very sentient to varying deleterious factors in the environment. Modern lifestyle with exposure to different chemicals may adversely affect the fertility parameters in female via their counteractive effect with estrogen (hormonal disruptive agents). The major hazard is difficult to predict owing to the consumption of various types of food additives whether in homes, restaurants, or processed food (Bojanić et al., 2009). According to our data, this research is considered the head most to comparatively evaluate both the individual and the combined toxic impacts of the flavoring agents (MSG) and artificial sweetener (ASP) on female reproductive functions.
In the current work, subchronic exposure to individual monosodium glutamate and ASP noteworthy increased body weight after repeated oral administration for 30 days. This result was similar to Oluba et al. (2011), who found that all MSG treated groups gained weight. This boosts the fact reported by Samuels (1999) that monosodium glutamate ingestion gives rise to an increase in the food zest which leads to increased body weight and adiposity. Also, Matyskova et al. (2008) had attributed the obesity resulted from MSG consumption to impaired sensitivity to leptin and insulin due to affection of the hypothalamic arcuate nucleus region which are associated with hyperleptinemia and hyperinsulinemia.
Also, Helal et al. (2019) and De Matos Feijó et al. (2013) observed an increase in body weight in ASP treated group. This finding may be attributed to an increase in the eagerness by the metabolites of ASP through various pathways. Phenylalanine is a metabolic product of ASP which may promote feed consumption by the hypothalamic adrenergic receptor and stimulating appetite centrally.
Aspartate, a metabolic product of ASP, reaches the arcuate nucleus and may be responsible for production of neuropeptide Y that can stimulate and increase carbohydrate consumption. Besides, it acts as an agonist on N-Methyl-D-aspartate receptors which also could increase food and fluid intake. Furthermore, ASP by itself could lower the level of leptin resulting in increased craving (Helal et al., 2019).
Our results revealed significantly declined estrogen levels in MSG treated group which was time-dependent. While ASP treated group (III) showed no significant changes in the serum level of estrogen. This result was in accordance with El-Beltagy and Elghaweet (2016) who observed that MSG administration in female rats led to a decrease in the level of estrogen in female rats. Bachmann et al. (2000) stated that the lowered estrogen levels induced depleted secretion of glycogen from the mucosa of the vagina and endometrium and representing the structural evidence for estrogen depletion process.
Subchronic exposure of MSG for 30 days induced a significant elevation and decrease of oxidative stress and antioxidant markers, respectively. Also, ASP treated group (III) showed the same result. This finding was in line with Diniz et al. (2004); Onyema et al. (2006) who observed elevated lipid peroxidation in rats exposed to MSG.
The foremost marker of oxidative stress is lipid peroxidation which is provoked by reactive oxygen species and leads to failure of different membrane functions (Selvakumar et al., 2006). And its boost in the present work could be due to the effect of increased production of ROS which is related directly to MSG administration. The observed decreases in antioxidant parameters occur due to the tissues try to preserve the normal oxidative status which may also induce lipid peroxidation of tissue (Okwudiri et al., 2012). Also, it can be explained by excitotoxicity which can be mediated via glutamate receptors and through ROS mediated oxidative stress (Xu et al., 2015).
Furthermore, the reports of Mourad and Noor (2011) supported our study observation. They documented that ASP administration at a dose of (40 mg/ kg) resulted in a noteworthy increase in liver tissue lipid peroxidation at the end of the 4th and 6th weeks. Furthermore, these results were consistent with Iyyaswamy and Rathinasamy (2012) who documented an increase of oxidative stress markers such as lipid peroxidation and superoxide dismutase in the rats’ brain on chronic treatment of ASP and this can be explained by elevated levels of blood methanol. Either methanol or its metabolites could initiate the oxidative damage.
In the present study, qRTPCR relative gene analysis showed that VASA mRNA expression was down-regulated in rats that received MSG, while not changed in ASP-treated group. Also, gene expressions of VASA were decreased significantly in the combined treated group (IV).VASA protein is a fundamental part of germplasm, not fully known congregation of RNA and proteins which is essential for the evaluation and function of germ cell. Expression of VASA was evidenced to balance with germ cell fate (Song et al., 2016). Accordingly, the function of VASA is crucial throughout oogenesis and spermatogenesis in matures as well as important during embryogenesis to specify the lineage of gametes. During development, VASA protein is uniquely noticeable in germ cells of both sexes (Castrillon et al., 2000). Identifying any mutation of VASA gene in humans could be of great concern; as such mutation may lead to sterility (Chen et al., 2016).
Histopathological assessment of ovaries of MSG- treated group (II) in our study showed some structural changes such as reduced maturity of graffian follicles and congested blood vessels. While ASP treated group (III) revealed no detectable changes. On the other hand, MSG+ ASP treated group (IV), showed marked atrophic and destructive changes of ovarian follicles and numerous congested, severely dilated blood vessels with a diffused hemorrhagic area in the medulla.
These results were near to Oladipo et al. (2015) who detected considerable structural changes as degenerated follicles and medulla with congested blood vessels in ovarian sections of rats treated with MSG for 14 days. Moreover, Eweka and Om’iniabohs (2011) observed that ovarian degenerative and atrophic processes were obvious with increasing MSG dosages. These degenerative ovarian changes may be explained by oxidative emphasize caused by MSG, which consistent with Ismail (2012); Al-Mosaibih (2013). The obvious ovarian pathologies and oxidative damage could be attributed to the ability of MSG to generate free radicals and oxygen reactive species which are serious for various biological systems due to interaction with DNA, proteins, and lipids leading to tissue damage (Singh and Ahluwalia, 2003). Furthermore, the dramaturgical impacts of monosodium glutamate on ovaries could be definite with the striking reduction in serum estrogen levels (Oladipo et al., 2015).
In addition, Eweka and Ominiabohs (2011) discussed that congestion of blood vessels within the ovarian medulla may be explained by the inhibited production of prostaglandin synthesis, which participates an important role in regulating circulation. These functional abnormalities usually lead to anovulatory infertility, which constitutes a major problem.
The histopathological assessment of pituitary gland in current study showed a cystic formation and few fibrotic bands in MSG treated group (II) while ASP treated group (III), showed only degenerated and necrotic areas with fragmented nuclei. However, marked disruptive structural fibrotic changes involving most of the pituitary anterior lobe were obvious in MSG+ ASP treated group (IV). These findings were near to Miskowiak and Partyka (1993) who documented a decrease in weight of pituitary glands when the dose of MSG (4 mg/g) was administered to newborn rats. These histopathological changes could be induced by liberated glutamate disintegrated out of monosodium glutamate; it could affect definite receptors located in central or peripheral neurons leading to degenerations of hypothalamic nuclei via disturbing the hypothalamus-pituitary-adrenal axis (Iamsaard et al., 2014).
Ochiogu et al. (2016) reported that adult rats and mice exhibited numerous endocrine and metabolic abnormalities when neonatally exposed to MSG and this could be attributed to selective neuronal damage of the hypothalamic.arcuate nuclei. Also,Tamura et al. (2002) discussed that the obvious shriveling of hypothalamic arcuate nucleus was detected in animals received MSG which had impaired the synthesis of growth hormone-releasing hormone resulting in short suture, atrophy of pituitary, gonad, and optical nerve. In the same context, Zelena et al. (1998) reported that consuming food additives such as MSG and ASP could reduce the relative weight of adrenal, pituitary, and thyroid glands. Moreover, Abdelhaliem and Mohamed (2017) reported that aspartame induced apoptotic changes in zona fasciculata cells of adrenal gland with subsequent corticotrophic hyperplasia. Apoptosis could be mainly explained via mitochondrial pathway due to the over-production of free radicals.
The present study revealed more deleterious impacts on some reproductive function of adult female Albino rats in group IV co-treated with (MSG+ ASP). As represented by exaggerated disruption of ovarian function and pituitary gland, which was evident by marked histopathological changes.
Interestingly, exposure to combinations of MSG or ASP is hazardous, maybe due tothe synergistic effects of interactions and cumulative potential of their individually caused toxicity. Abu-Taweel et al. (2016) confirmed our findings and documented marked oxidative stress induced by the combined administration of MSG and ASP. This might be one of the possible explanations for the structural changes and the subsequent dysfunctions in the ovary and pituitary of the tested animals in our study.
Conclusions
Finally, it could be concluded that the combined use of ASP and MSG is more significantly and synergistically dangerous on the ovary of adult albino rats than their individual use. The combined toxic impacts of the flavoring agents (MSG) and the artificial sweetener (ASP) still unclear and the underlying mechanism of the synergistic interactions and reinforcement of toxicity should be further investigated. Accordingly, we recommend conducting future research on the drawbacks of Commingle consumption of MSG and ASP on other organs are also much needed to understand their effects on other systems.
acknowledgements
The authors gratefully acknowledge the help and support provided by all the staff of animal House, Faculty of Medicine, Zagazig University.
Conflicts of interest
Authors declared non conflicts of interest.
authors contribution
All authors contributed to the study conception, design, material preparation, investigations, data collection and analysis. The first draft of the manuscript was written by all authors. All authors read, and approved the final manuscript.
References






