Advances in Animal and Veterinary Sciences
Case Report
Gross and Microscopic Study of Pneumonic Lungs, Liver and Intestine from Peste des Petits Ruminants (PPR) Suspected Dead Goat in Sylhet Region of Bangladesh
Md Mazedul Islam1, Abu Hena Mustafa Kamal1, Monira Noor1, Jamal Uddin Bhuiyan2, Real Datta2*
1Department of Pathology, Faculty of Veterinary, Animal and Biomedical Science; Sylhet Agricultural University, Sylhet, Bangladesh; 2Department of Parasitology, Faculty of Veterinary, Animal and Biomedical Science; Sylhet Agricultural University, Sylhet, Bangladesh.
Abstract | Four dead goats were collected at regional veterinary hospital, Sylhet, Bangladesh with previous history of anorexia for 3 days, diarrhoea, oculo-nasal discharge and high fever. At necropsy, congested and pneumonic lung, zebra striping in colon were characteristic findings in all four goat carcasses but stomatitis was observed in two goats who showed erosion in lips and gum during external examination. Liver lesions were not prominent upon external examination. Histopathological analysis revealed that, there were interstitial pneumonia along with sloughing of bronchial epithelium in lungs from all four goats. Prominent type II pneumocytes caused expansion of alveolar wall in all four goats. Out of four, only one goat was found with the presence of syncytial cell in the alveoli. Numerous inflammatory cells were found in the submucosa and lamina propria of intestinal samples collected from all four goats. Vacuolar degeneration and syncytial cells were observed in hepatocytes of liver samples from two goats. Periportal multifocal lymphocytic infiltrations were common in liver parenchyma. Gross and microscopic lesions were similar to the previous pathomorphological studies of PPR conducted by several other authors, which validate the present cases of suspicion of PPR infection.
Keywords | PPR, Goat, Sylhet, Gross Pathology, Histopathology.
Received | September 24, 2020; Accepted | September 30, 2020; Published | January 01, 2021
*Correspondence | Real Datta, Department of Parasitology, Faculty of Veterinary, Animal and Biomedical Science; Sylhet Agricultural University, Sylhet, Bangladesh; Email: rdatta.parasitology@sau.ac.bd
Citation | Islam MM, Kamal AHM, Noor M, Bhuiyan JU, Datta R (2021). Gross and microscopic study of pneumonic lungs, liver and intestine from peste des petits ruminants (ppr) suspected dead goat in sylhet region of bangladesh. Adv. Anim. Vet. Sci. 9(2): 315-319.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.315.319
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Datta et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Peste des petits ruminants (PPR) is a highly contagious and economically important viral disease of domestic and wild small ruminants which is caused by the genus morbillivirus under the family Paramyxoviridae (Bailey et al. 2005). In Bangladesh, PPR is an exotic disease of goats (Debnath, 1995) which was first described in Bangladesh by FAO expert team in 1993 (Dhar et al., 2002) and the causal agent of the disease was identified as PPR virus by World Reference laboratory in the U.K (Dhar et al., 2002).
According to the FAO estimates, the morbidity, mortality, production losses and treatment cost of PPR altogether are likely to cause an economic loss of $2,972.5 million/year during 2012-2017 in SAARC region among which, in India alone, it would be $2569.00 million/year (Felix, 2013). While in Bangladesh, the estimated economic loss in 2011 due to PPR was reported as Tk 10, 16434 ($ 14520.49) (Islam et al., 2011). Though, this disease is considered as one of the hindrance of poverty alleviation program in African and Asian poor countries since sheep and goat rearing contributes significantly in the livelihood of rural poor families (Diallo, 2006), very few researches were carried out about this viral infection in Bangladesh. And no known pathological study was found conducted in Sylhet region till the period when this study was carried out. Moreover, due to lack of sufficient facilities, veterinary hospitals rely on only external examination to diagnose this disease. In this study we aimed to describe the gross and histopathological features found in PPR suspected goat samples. We collected four dead goats that were supposedly died of PPR viral infection at different days in January 2014, with the clinical features which were used to make a tentative diagnosis to put to the hospital records, then conducted gross examination and histopathological analysis of affected organs and found the lesions identical to the PPR.
Materials and Methods
Gross Examination
Four dead goats (1 boar goat of African origin and 3 were Black Bengal Goat) were collected from the regional veterinary hospitals in January 2014 suspected with PPR viral infection. Upon receiving in the laboratory, physical examination was conducted to record external clinical features followed by necropsy in each goat. Necropsy was conducted as per standard protocol (King et al., 2013). Various organs were dissected out aseptically and moved onto trays for closer inspection and processing. Lung, liver and intestine were carefully examined to detect congestion and haemorrhage. Then the digestive organs were opened through longitudinal incision from rumen to terminal colon to detect the lesion in the digestive system. Lymph nodes were collected to check the lymphatic system.
Histopathology
For histopathological study, every tissue sample (lung, liver and intestine) was labelled using cotton string and white paper with name of specific organ for identification. Tissues were cut into small pieces of approximately 2x3 to 2x4 sq. mm using surgical knife for making suitable block. Tissues were preserved and fixed immediately after collection in 10% formalin for 7 days. Following fixation, the procedures were followed as described by Kiernan (1992). Haematoxylin and Eosin was used for staining the slides. Following the staining, the slides were mounted one by one. Canada balsam was used as a mounting medium. Histopathological slides were examined using bright field light microscope with 10x and 40x magnification. Images were captured by using USB bright field light photomicroscope.
Results and Discussion
Gross pathological findings
In the present study, watery, mucopurulent ocular and nasal discharge, erosion and ulceration of the oral mucosa with encrustation of the nostril were present in
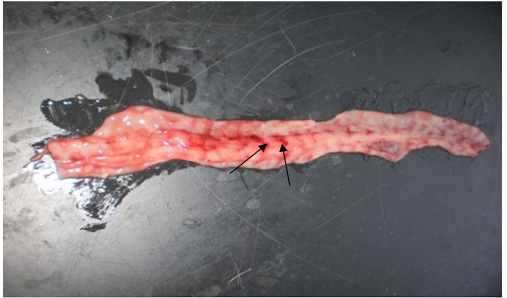
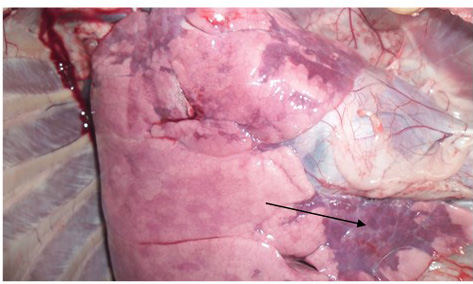
Figure 3: Zebra striping in colon
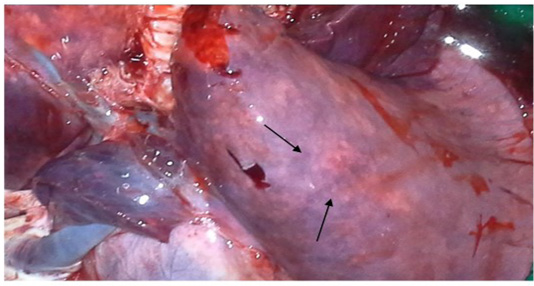
two goats out of four, while diarrhoea was common in all four goats; the features were similarly found by Mabdoli & Ali (2012), Yener et al. (2004) and Rashid et al. (2008). At necropsy, most prominent lesions were found in respiratory system. There were pneumonic lungs with congestion and consolidation in all four goats particularly the cardiac lobe of lungs was mostly affected as consolidated with dark red discoloration (Figure 1 & 2). Frothy mucus was observed in cut pieces of lungs on squeezing. The involvement of respiratory system in PPR is remarkable and pneumonia is a predominant sign in PPR. Most of the previous reports found lung involvement in almost more than 90% of cases dying during outbreak of PPR (Tripathi et al., 1996; Kumar et al., 2004). Our findings are also in agreement with
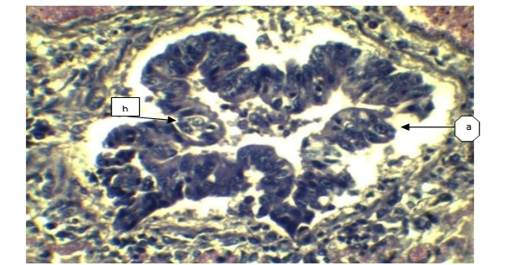
Figure 4: a) Sloughing off surface epithelium of bronchi; b) Necrosis of bronchiolar epithelium. (H&E x 40).
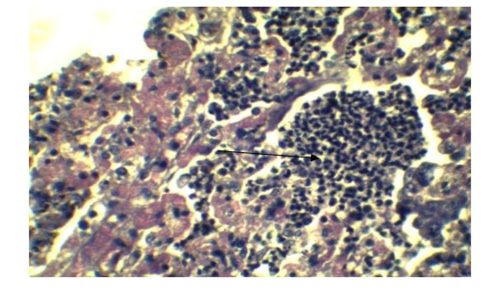
Figure 5: Infiltration of inflammatory cell in Lung parenchyma (Arrow), feature of interstitial pneumonia. (H&E x 40)
the previous reports of Khan et al. (2005) and Rahman et al. (2011). The intestinal mucosa was congested, hemorrhagic and covered with mucous in all four goats which were similar with the previous results of Kumar et al. (2004) and Rahman et al. (2011). Most striking lesion was found at large intestine especially in the posterior part of colon and rectum, where discontinuous streaks of congestion (‘‘Zebra’’ stripes or ‘‘Zebra markings’’) on the mucosal folds were observed (Figure 3) which is in agreement with the findings by Rahman et al. (2011). In liver, lesions were not so prominent except the presence of congested line on the liver surface from two goats. Although there was no report of such liver lesion found in previous studies, but Kul et al. (2007) reported multifocal coagulation necroses in the liver which was not found in the present study. However, our gross pathological findings are considered as recognizing sign of PPR by FAO (Roeder and Obi, 1999; Vinayagamurthy, 2017).
Histopathological findings
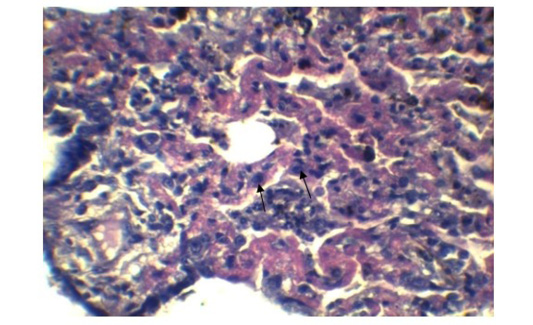
In lung section histopathology, we found necrosis and sloughing of surface epithelium, infiltration of mononuclear cell in bronchi and bronchiole. Sloughing of surface epithelium and infiltration of mononuclear cells in alveoli, bronchi and bronchiole were the common findings (Figure 4 & 5). Necrosis of bronchial epithelium was also observed by Emikpe et al. (2010) and Mabdoli & Ali (2012).
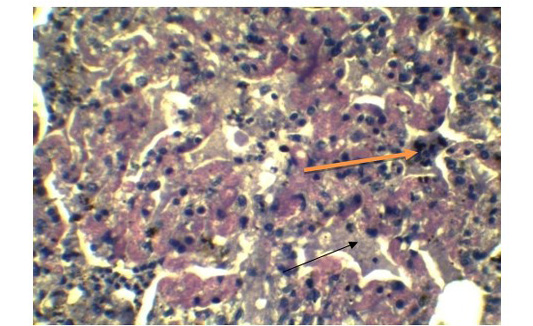
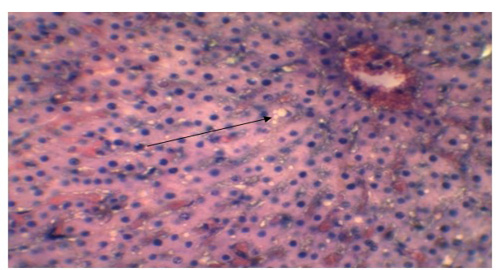
Figure 7: Presence of syncytial cell in the alveoli (Yellow arrow). Presence of fibrin exudates in parenchyma (Black arrow) (H&E x 40).
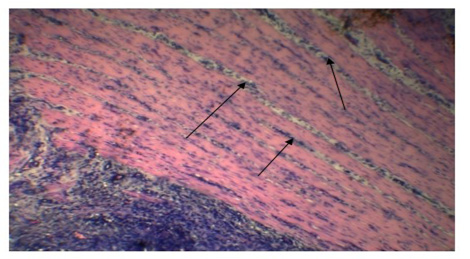
Alveolar wall expansion by hyperplastic type-II pneumocytes (Figure 6) were similarly found by Truong et al. (2014). Type-II pneumocytes are larger cuboidal cells can be distinguished from thin, squamous Type-I pneumocytes. Congestion with infiltration of reactive cells within interstitium, lumen of alveoli, bronchi and bronchiole, inflammatory exudates containing large amount of fibrin accumulated over a considerable area of parenchyma were characteristic to pneumonic lung (Figure 7). These features were similarly reported in PPR previously by Truong et al. (2014) and Rashid et al. (2008). Presence of syncytial cell in the alveoli (Figure 7) is consistent with the previous findings (Kumar et al., 2004; Kul et al., 2007) which is considered as pathognomic to PPR by Kul et al. (2015).
In our findings intestinal submucosa was diffusedly infiltrated with inflammatory cells (Figure 8), villi were destructed due to necrosis; which were consistent with the findings of Khan et al. (2005) and Madboli & Ali, (2012), who also observed coagulative necrosis of intestinal villi. Kumar et al. (2004) and Truong et al. (2014) observed necrosis and degeneration of intestinal epithelium in PPR affected goat, which is relevant with the present study. In liver, the characteristic finding was the presence of vacuolar degeneration (Figure 9) in hepatocyte which is in accordance with the report of Madboli and Ali, (2012). However, we presented limited samples and limited findings in this report that might raise the question of data sufficiency, but there is no doubt that the features we identified during gross and microscopic examinations were valid as per previous authors, which suggest the importance of more robust examination using large number of samples. Though we could not confirm the virus due to our limitations in the laboratory equipment’s, our observed findings were considered as recognizing features of PPR by FAO (Roeder and Obi, 1999; Vinayagamurthy, 2017). Gross and microscopic lesions we found in this study are more or less similar to the findings of other previous authors and some of them are considered as pathognomic to PPR, which indicates that the suspected cause of the death of goats supposedly due to the consequence of PPR viral infection.
Conclusions
In the present study, we observed diarrhoea, oculo-nasal discharge, encrustation of the lips and gum upon external examination of PPR suspected goats. At necropsy, congested and consolidated pneumonic lung, and zebra striping of colon were the characteristic gross pathology. Histopathological analysis revealed infiltration of mononuclear cells in lung tissue sections, hyperplastic type-II pneumocytes, vacuolar degeneration in liver section, infiltration of inflammatory cells in intestinal mucosa. The lesions were identical to PPR as described by various previous authors. This report will help to improve the confidence of Veterinary Practitioner’s in this region to diagnose the disease in limited diagnostic facilities. It will also assist the future researchers to validate the clinical findings of PPR in Goat in this region. However, to confirm the virus, immune based detection is necessary. Till date, there is no comprehensive study conducted on the exact economic impact of PPR in any region of Bangladesh which is necessary in respect to poverty alleviation program. The high prevalence of the viral infection also demands the molecular characterization of viral strain in this region to develop more effective vaccine with high accuracy.
Acknowledgments
The authors are highly thankful to the Department of Pathology, Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Sylhet for their cooperation and provision of research facilities.
Conflict of Interests
The authors declared that there is no conflict of interests.
Authors Contribution
All authors contributed equally.
References