Advances in Animal and Veterinary Sciences
Research Article
Effects of Melatonin on Cryopreserved Semen Parameters and Apoptosis of Thai Swamp Buffalo Bull (Bubalus bubalis) in Different Thawing Conditions
Subash Chandra Chaudhary1,2, Niran Aeksiri1,2, Amornrat Wanangkarn1,2, Yu-Jing Liao3, Wilasinee Inyawilert1,2*
1Department of Agricultural Science, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok, 65000, Thailand; 2The center for Agricultural Biotechnology, Naresuan University, Phitsanulok, 65000, Thailand; 3Livestock Research Institute, Council of Agriculture, Executive Yuan, Tainan, 71246, Taiwan.
Abstract | Semen cryopreservation process leads to over production of reactive oxygen species (ROS) compromising the semen quality. Melatonin is a neuroendocrine hormone which acts as an antioxidant to protect the cells from oxidative stress. Hence, the present study was conducted to investigate the effects of melatonin supplemented to the semen extender on the cryopreserved semen quality parameters and apoptosis of swamp buffalo semen after thawing with different methods. Semen, collected from four bulls by artificial vagina, was divided into two equal aliquots, diluted with (0 mM and 1 mM) melatonin supplemented tris-egg yolk-citrate-glycerol extender, and finally cryopreserved in liquid nitrogen. Two different thawing methods were performed: 30°C for 60 seconds and 37°C for 30 seconds. The computer assisted semen analysis results showed that the percentage of total motility and progressive motility in the melatonin treated samples were significantly higher (p ˂ 0.01) than those in the untreated groups at both thawing methods. All the sperm kinetic parameters, excluding the wobble, were higher in the samples treated with melatonin and thawed at 37°C for 30 seconds. The flow cytometry results showed that there was lower apoptotic sperm in all thawing conditions in the samples treated with melatonin. The percentage of apoptotic spermatozoa was found significantly lower (p < 0.01) in the treated samples using 37°C for 30 seconds of thawing procedure than that in the samples when thawed at 30°C for 60 seconds. Hence, the overall results of the present study confirmed that 1 mM melatonin supplementation to the semen extender can improve the motility and viability of spermatozoa and reduce the number of apoptotic sperm in the frozen-thawed semen of swamp buffalo.
Keywords | Apoptosis, Melatonin, Semen Quality, Swamp Buffalo
Received | October 14, 2020; Accepted | October 22, 2020; Published | January 01, 2021
*Correspondence | Wilasinee Inyawilert, Department of Agricultural Science, Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok, 65000, Thailand; Email: wilasineei@nu.ac.th
Citation | Chaudhary SC, Aeksiri N, Wanangkarn A, Liao YJ, Inyawilert W (2021). Effects of melatonin on cryopreserved semen parameters and apoptosis of thai swamp buffalo bull (bubalus bubalis) in different thawing conditions Adv. Anim. Vet. Sci. 9(2): 238-245.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.238.245
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Inyawilert et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Good quality of cryopreserved semen is required to get positive artificial insemination outcomes (Oliveira et al., 2012). However, the quality of semen can be compromised by various factors such as cryopreservation process (Rasul et al., 2001; Bailey et al., 2003; Khan et al., 2009), types of extender used (Crespilho et al., 2012; Harahap et al., 2020) and season of semen collection (Koonjaenak et al., 2007; Dessouki et al., 2011). During the cryopreservation process, reactive oxygen species (ROS) are excessively generated (Wang et al., 1997) in human sperm. ROS causes lipid peroxidation on the sperm plasma membrane (da Silva Maia et al., 2010) impairing membrane fluidity and the activity of membrane enzymes and ion channels and therefore resulting in poor sperm motility and fertility (Pons-Rejraji et al., 2009). Moreover, a higher percentage of apoptotic spermatozoa have been observed due to the cryopreservation procedures (Martin et al., 2004; Khan et al., 2009; Kaur and Atreja, 2018) and is associated with the cell death, increased membrane permeability, DNA damage, and reduced fertility (Anzar et al., 2002; Wang, 2003; Martin et al., 2004; Martin et al., 2007). One of the main causes of increased apoptotic spermatozoa is excessive ROS and oxidative stress (Carmody and Cotter, 2001; Wang, 2003). Moreover, the indigenous antioxidants are dramatically reduced during freeze-thaw cycle (Bilodeau et al., 2000) and the buffalo sperm is more susceptible to oxidative stress than cattle sperm (Nair et al., 2006).
Adding antioxidants to the semen extender can enhance the quality of cryopreserved semen (Foote et al., 2002; Rather et al., 2016). Melatonin (N-acetyl-5-methoxytryptamine) is a neuroendocrine hormone secreted rhythmically from the pineal gland and regulates various physiological functions, such as circadian cycle (Kennaway and Wright, 2002), seasonal reproduction in photoperiodic species, (Reiter et al., 2003), anticancer effects (Di Bella et al., 2013), immune system and aging (Srinivasan et al., 2005). Besides, melatonin has a natural antioxidant property and protects the cells from oxidative stress by promoting oxygen radical scavenging activities by scavenging free radicals and interacting with antioxidant enzymes (Deng et al., 2017). Melatonin has been used as an antioxidant in the semen of different mammals for the improvement of the post-thawed semen quality (Succu et al., 2011; Ashrafi et al., 2013; El-Raey et al., 2015; Karimfar et al., 2015). Thus, considering its beneficial effect on semen quality, this study aimed to investigate the effects of melatonin supplementation to semen extender on the cryopreserved semen parameters and apoptosis of spermatozoa of Thai swamp buffalo bull at the different thawing methods.
MATERIALS AND METHODS
Semen collection and processing
Four swamp buffalo breeding bulls (4 years to 6 years of age), maintained at HJ Buffalo Farm, Uthai Thani (Thailand) under the same feeding, housing, and the other management system, were used for the semen collection. Each semen ejaculate was collected with the aid of a pre-warmed artificial vagina (42°C). Semen was evaluated initially for the volume, color, and presence of any impurities immediately after collection and kept in the water bath at 37°C until the estimation of initial motility and concentration. The ejaculate with the minimum standards of concentration > 500 x 106 spermatozoa/mL and initial motility > 70% were used. Tris citric acid egg yolk extender containing 200 mM tris-(hydroxymethyl)-aminomethane, 70 mM citric acid, 50 mM fructose, 20% (v/v) egg yolk, 6.4% (v/v) glycerol, benzyl penicillin (1,000 IU/mL), and streptomycin sulfate (1000 µg/mL) was used as the freezing extender. Melatonin (M5250; Sigma-Aldrich, St. Louis, MO, USA), dissolved in 0.1% dimethyl sulfoxide (DMSO), was added to the extender to provide the final concentration of 1.0 mM. One-half of the aliquot as the treatment group was diluted with the extender containing 1.0 mM melatonin and the other one-half of the aliquot was diluted with the normal extender but containing 0.1 % DMSO as well. The diluted semen was then filled in the 0.25 mL straws (IMV Technologies, France) and wrapped in the cotton cloth to cool slowly at 4°C for two hours and unwrapped and equilibrated at 4°C for another two hours. Finally, the racks with the semen straws were kept 5 cm above the liquid nitrogen for 15 minutes and stored in the liquid nitrogen tank.
Thawing methods
We performed two methods to thaw the frozen semen straws in the water bath: 30°C for 60 seconds and 37°C for 30 seconds. These two thawing methods were executed in both control and melatonin groups.
Assessment of sperm motility parameters
After thawing the frozen semen straws as mentioned above, the aliquots of 3 µL of thawed semen in the control and melatonin groups were pipetted and loaded into pre-heated slides (Leja Slides, SCA®, Spain) at 37°C. The semen motility and kinetic parameters were measured using CASA (Androvision® CASA Software Version 1.0.0.9, Minitube, Germany). The total motility, progressive motility, straight-line velocity (VSL), curve-line velocity (VCL), average path velocity (VAP), distance average path (DAP), distance straight line (DSL), distance curve line (DCL), linearity (LIN), straightness (STR), beat cross frequency (BCF), wobble (WOB), and amplitude of lateral head displacement (ALH) were measured.
Assessment of apoptotic spermatozoa by Annexin V/ 7 AAD staining
Guava Nexin® Reagent (4500-0450, Merck KGaA, 64271, Darmstadt, Germany) containing annexin V and 7 AAD was used to assess apoptosis according to the manufacturer’s instruction with slight modification. Briefly, after thawing the frozen straw, the semen was transferred into 1.5 mL Eppendorf tubes and centrifuged at 1,000 x g for 10 minutes to remove egg yolk extender. After washing with phosphate-buffered saline (PBS) twice, the samples were centrifuged again at 1,000 x g for 5 minutes. Then, the sperms were diluted to ≤ 2 x 105 cells/mL in PBS. Finally, the sperms were incubated with Guava Nexin® Reagent for 20 minutes in dark. The percentage of apoptotic sperm was assessed using a flow cytometer (Guava® easyCyte 5HT HPL, Merck KGaA, 64271, Darmstadt, Germany).
Table 1: Mean (±SEM) percentages of sperm motion parameters of frozen-thawed buffalo bull spermatozoa with melatonin treatment at different thawing methods
| Sperm Parameters | Control | Melatonin 1mM | |||
| Thawing at 30 °C for 60 Sec | Thawing at 37 °C for 30 Sec | Thawing at 30 °C for 60 Sec | Thawing at 37 °C for 30 Sec | ||
| T M (%) |
35.12b ± 1.66 |
35.88b ± 1.54 |
40.59a ± 1.89 |
43.18a ± 1.62 |
|
| P M (%) |
29.18b ± 1.72 |
28.05b ± 1.58 |
33.30a ± 1.75 |
35.87a ± 1.54 |
|
| VCL [µm/s] |
54.16b ± 2.43 |
51.60b ± 2.11 |
55.20a ± 2.45 |
59.69a ± 1.60 |
|
| VSL [µm/s] | 24.88 ± 1.74 | 22.52 ± 1.30 | 22.86 ± 1.52 | 27.07 ± 1.34 | |
| VAP [µm/s] | 30.03 ± 1.78 | 27.68 ± 1.41 | 28.34 ± 1.57 | 32.38 ± 1.27 | |
| DCL [µm] | 23.54 ± 1.12 | 22.61 ± 0.88 | 24.325 ± 1.05 | 25.65 ± 0.90 | |
| DSL [µm] | 9.79 ± 0.78 | 8.91 ± 0.52 | 9.04 ± 0.65 | 10.99 ± 0.59 | |
| DAP [µm] | 12.22 ± 0.80 | 11.37 ± 0.56 | 11.67 ± 0.67 | 13.33 ± 0.58 | |
|
ALH [µm] |
0.65 ± 0.02 | 0.63 ± 0.02 | 0.66 ± 0.02 | 0.98 ± 0.29 | |
| BCF [Hz] | 8.07 ± 0.61 | 7.06 ± 0.38 | 7.66 ± 0.44 | 9.16 ± 0.47 | |
| HAC [rad] | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.15 ± 0.01 | 0.37 ± 0.21 | |
| LIN | 0.44 ± 0.01 | 0.43 ± 0.01 | 0.41 ± 0.02 | 0.45± 0.01 | |
| STR | 0.81 ± 0.01 | 0.80 ± 0.01 | 0.79 ± 0.01 | 0.82 ± 0.01 | |
| WOB | 0.54 ± 0.01 | 0.53 ± 0.01 | 0.51 ± 0.01 |
0.53± 0.01 |
|
TM: total motility, PM: progressive motility, VCL: velocity curved line, VAP: velocity average path, VSL: velocity straight line, LIN: linearity coefficient (VSL/VCL), STR: straightness coefficient (VSL/VAP), BCF: beat cross frequency, ALH: amplitude of lateral head displacement, DCL: distance curve line, DSL: distance straight line, DAP: distance average path, WOB: wobble (VAP/VCL) a,b values with different superscript letters in each raw represent statistically significant differences (p < 0.05). Number of replicates for each group (n) = 40
Statistical Analysis
The experimental data were statistically analyzed by using Statistical Product and Service Solutions (SPSS 19.0 for windows; SPSS, Chicago, IL, USA). Analysis of variance was done by general linear model - two way between groups to assess the impact of melatonin treatment and thawing methods on the various dependent variables. The data were presented as mean ± standard error of the mean, and p value less than 0.05 indicated a significant difference.
RESULTS
Effect of melatonin on sperm motility characteristics with the different thawing methods
The effects of melatonin supplemented to the extender on the semen motility parameters are shown in Table 1. The total motility, progressive motility, and VCL of spermatozoa in the samples supplemented with 1 mM of melatonin were higher (p < 0.05) than those in the control groups in both thawing conditions. However, the velocity parameters such as VSL, and VAP showed no significant differences between the treatment groups and also between the different thawing methods. The distance parameters, such as DCL, DSL, and DAP, and the parameter ALH, BCF, LIN, STR, and WOB also showed no significant differences between the control and melatonin treated samples. All the motility parameters, excluding WOB, were higher when melatonin treated frozen straws were thawed at 37°C for 30 seconds.
The effect of melatonin in apoptosis of spermatozoa with the different thawing methods using flow cytometry with annexin V/ 7AAD
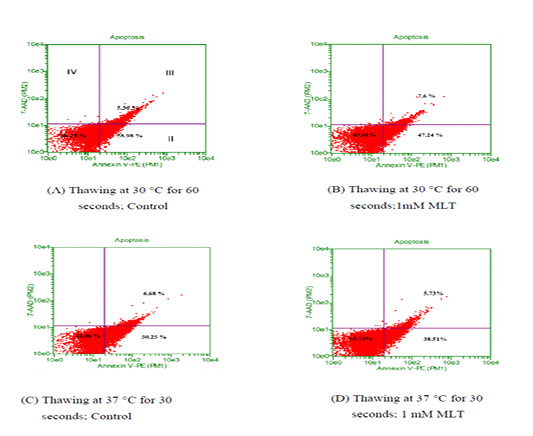
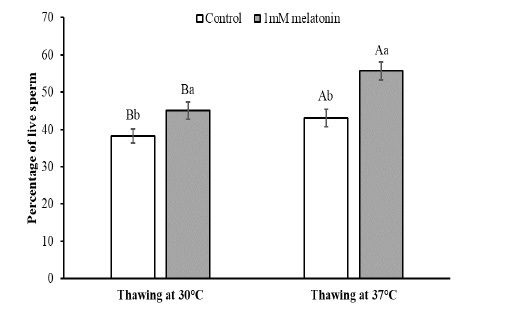
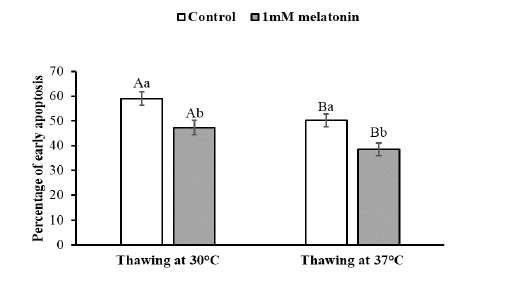
To observe the effects of melatonin supplementation on the sperm apoptosis, semen straws were thawed with two different temperature and time combinations and stained with annexin V/7 AAD reagent. The flow cytometry dot plots of spermatozoa for the control and melatonin groups in different thawing conditions are shown in Figure 1. The flow cytometry dot plots, as shown in Figure 1-A, have four quadrants: (I) the percentage of live spermatozoa (II) the percentage of early apoptotic spermatozoa; (III) the percentage of late apoptotic/dead spermatozoa; (IV) the cell debris. The bar graphs depicting the percentage of live spermatozoa and early apoptosis in different thawing conditions are shown in Figure 2 and Figure 3. The percentage of live spermatozoa in the group treated with 1 mM melatonin was significantly higher (p < 0.01) than the control groups in both thawing conditions. Within the melatonin groups, the highest percentage of live sperm (55.74% ± 2.41%) was found when thawed at 37°C for 30 seconds. This result was significantly higher (p < 0.05) than that of thawing at 30°C for 60 seconds (45.01% ± 2.30%). However, the percentage of early apoptosis was significantly lower (p < 0.01) in the melatonin treated samples than control samples. The lowest percentage of early apoptotic spermatozoa (38.51% ± 2.55%) was found in the melatonin group when thawed at 37°C for 30 seconds. This result was significantly lower (p < 0.05) than thawing at 30°C for 60 seconds (47.24% ± 2.99%).
Thawing at (A-B) 30 °C for control and treatment group ;(C-D) 37 °C control and treatment group. Events in the lower-left quadrant represent annexixn V –ve/7AAD –ve spermatozoa (viable sperm), and events in the lower-right quadrant represent early apoptotic spermatozoa (annexin V +ve /7AAD -ve). Spermatozoa in the upper-right quadrants represent late apoptotic/dead cells (annexin V+ve /7AAD +ve)
The data expressed as Mean ± SEM. Different letters in each bar represent statistically significant differences (p < 0.01) where (a, b) represent difference between treatment groups and (A, B) represent difference between thawing groups.
The data expressed as Mean ± SEM. Different letters in each bar represent statistically significant differences (p < 0.01) where (a, b) represent difference between treatment groups and (A, B) represent difference between thawing groups.
DISCUSSION
During the freezing-thawing process, the semen quality might be lost by 40 % to 50 % caused by excessive ROS formation leading to oxidative stress (Bilodeau et al., 2000; Zhang et al., 2015). Melatonin, a powerful antioxidant, has been used to improve the semen quality of different species (Balao da Silva et al., 2011; El-Raey et al., 2014; Deng et al., 2017; ChaithraShree et al., 2019). Although some studies have been done in river buffaloes, no such study has been done in swamp buffalo bull semen. Therefore, the present study was commenced to investigate the effects of melatonin on the frozen-thawed semen quality of swamp buffalo in different thawing conditions.
In the present study, the motility parameters of spermatozoa, such as the percentage of total motility and progressive motility, were improved when the semen extender was supplemented with melatonin. These results indicated that the swamp buffalo bull semen supplemented with the melatonin yielded superior semen quality than untreated semen. These findings are in accordance with other studies with melatonin supplementation in bull semen (Ashrafi et al., 2013; Kapadiya et al., 2016; ChaithraShree et al., 2019), buffalo bull semen (El-Raey et al., 2014; El-Raey et al., 2015; Abdel-Khalek et al., 2016), mithun semen (Perumal et al., 2013; Perumal et al., 2016; Perumal et al., 2018), ram semen (Ashrafi et al., 2011; Succu et al., 2011), and human semen (Karimfar et al., 2015; Deng et al., 2017) to improve the semen motility. These positive contributions for sperm motility offered by melatonin have been explained by different authors in different ways. According to Succu et al. (2011), Ashrafi et al. (2013), Karimfar et al. (2015), Chen et al. (2016) and Deng et al. (2017) melatonin supplementation, by decreasing the ROS level and the lipid peroxidation, avoid the oxidative damage to the spermatozoa membrane and maintain the sperm motility. According to El-Raey et al. (2015), melatonin can preserve and maintain the ultrastructure integrity of the plasma membrane and mitochondrial arrangement of the cryopreserved sperm so that the sperm are less susceptible to the cryo-injury, and thus the motility can be maintained. Melatonin has also shown the positive effects on the antioxidant enzymes, such as superoxide dismutase (SOD) and glutathione, by increasing their expression to improve the total antioxidant capacity (El-Raey et al., 2014; Deng et al., 2017) resisting the oxidative damage. Some studies showed that melatonin has the protective effect on preventing oxidative damage in mitochondria by maintaining the oxidative phosphorylation and ATP production (López et al., 2009), and thus higher intracellular ATP concentrations could be observed (Succu et al., 2011). These all positive effects might be the possible causes for the increase in the motility of the spermatozoa with the extender supplemented with melatonin.
Some studies have revealed that apoptosis of spermatozoa is increased during the freezing-thawing process (Martin et al., 2004; Martin et al., 2007; Khan et al., 2009). The externalization of phosphatidylserine (PS) from the inner leaflet to the outer leaflet of the plasma membrane occurs during the process of apoptosis (Martin et al., 1995; Verhoven et al., 1995). Annexin V, the calcium-dependent phospholipid-binding protein is used to study apoptosis because of its high affinity for PS (Koopman et al., 1994). In this study, we used annexin V/7-AAD to detect apoptotic spermatozoa and the late apoptotic/dead spermatozoa with flow cytometry. 7-AAD is a membrane-impermeable fluorescent DNA intercalator. Therefore, late-stage apoptotic and dead cells will be stained with annexin V/7-AAD but not the live, healthy, and early apoptotic cells. In the present study, the percentage of early apoptotic spermatozoa in the melatonin treated frozen-thawed semen was found significantly lower (p < 0.05) than the untreated frozen-thawed semen in all thawing conditions. This indicates that the melatonin supplemented in the semen extender can enhance the semen quality by reducing the apoptotic effects in the frozen-thawed semen. Similarly, when melatonin was supplemented in the semen extender, reduced percentage of apoptotic spermatozoa was observed in the various animal species such as mithun semen (Perumal et al., 2018), ram semen (Casao et al., 2010), rooster semen (Mehaisen et al., 2020), stallion spermatozoa (Balao da Silva et al., 2011), and human spermatozoa (Espino et al., 2010; Deng et al., 2017). The decrease in apoptotic spermatozoa in the present study might be due to the strong anti-oxidative capacity of melatonin to diminish the oxidative stress by reducing the levels of intracellular ROS (Karimfar et al., 2015; Deng et al., 2017; Najafi et al., 2018) protecting from lipid peroxidation (Ashrafi et al., 2013). Melatonin also increases the expression of Nrf2 (Deng et al., 2017) and the expression of antioxidant enzymes such as superoxide dismutase, catalase, and heme oxygenase-1 (Ashrafi et al., 2013). These effects eventually increase the antioxidant capacity in the frozen-thawed sperm. Melatonin is also able to reduce the pro-apoptotic gene Bax and increase the expression of anti-apoptotic gene Bcl-2 (Chen et al., 2016; Deng et al., 2017). Thus, the anti-apoptotic effect in the melatonin treated semen might result from these all combined mechanisms.
We also attempted to evaluate the different thawing temperatures and time combinations with the semen quality. Thawing procedure is also equally important as the freezing procedure affecting the survival of spermatozoa (Nur et al., 2003) because during freezing and thawing procedures spermatozoa have to pass through the critical temperature zone (-5°C to -50°C) causing detrimental effects on survival (Borah et al., 2015). In this study, the thawing of frozen semen at 37°C for 30 seconds illustrated the best live percentage of spermatozoa. At this thawing condition, the apoptotic spermatozoa were also significantly lower (p < 0.05), and the live percentage of spermatozoa higher when compared with thawing at 30°C for 60 seconds. Moreover, the motility characteristics, as obtained by CASA evaluation, were also the best. These findings were in line with the results from previous studies (Rao et al., 1986; Correa et al., 1997; Nur et al., 2003; Chaiprasat et al., 2006; Nur et al., 2006; Al-Badry, 2012). Hence, thawing at 37°C for 30 seconds was considered as the best thawing procedure. Similarly, Dudeja (1990) found that the thawing of frozen semen in water-bath at 35°C for 30 seconds yielded satisfactory post-thawed viability and motility. However, many of the researchers (Robbins et al., 1976; Nur et al., 2003; Rastegarnia et al., 2013; Borah et al., 2015; Lyashenko, 2015) have found out that thawing at a higher temperature (from 60°C to 80°C) for short duration yields better post-thawed motility and viability.
CONCLUSION
The overall results of the present study confirm that melatonin supplementation to the semen extender can improve the motility and viability of spermatozoa and decrease the apoptotic sperms in the frozen-thawed semen of swamp buffalo. Furthermore, the semen quality was better when the frozen straws were thawed at 37°C for 30 seconds.
ACKNOWLEDGEMENTS
The authors acknowledge the Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Phitsanulok, Thailand; Thailand International Cooperation Agency (TICA) for providing the scholarship and fund; HJ Buffalo Farm, Uthai Thani (Thailand), and all the helping hands to accomplish this research.
CONFLICT OF INTEREST
There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
W Inyawilert designed the experiment, S C Chaudhary performed the experiment, S C Chaudhary and W Inyawilert analyzed the data, S C Chaudhary drafted the manuscript, W Inyawilert, N Aeksiri, A Wanangkarn, and Y-J Liao revised and edited the manuscript.
COMPLIANCE WITH ETHICAL STANDARDS
The use of animals and the procedures for animal handling was approved by the Institutional Animal Use and Care Committee (IACUC) at Naresuan University, Thailand (no. NU-AEE620506).
REFERENCES