Advances in Animal and Veterinary Sciences
Short Communication
Stocking Density Associated with Parasitic Infection by Gastrointestinal Examination in Laboratory Mice
Raslan Ain-Fatin1, Saulol Hamid Nur-Fazila1*, Md Isa Nur-Mahiza1, Abd Rahaman Yasmin2, Yong Meng Goh3
1Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; 2Department of Veterinary Laboratory Diagnostics, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; 3Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia.
Abstract | Parasitic infection in the laboratory mice (Mus musculus) may affect certain research outcomes especially during high worm burden. This study allows the assessment of parasite infection of mice based on stocking density that potentially serve as a guideline for proper management to be implemented. Fifty-four (54) male BALB/c mice were randomly chosen from an animal facility located in Klang Valley, Malaysia and placed for three replicates in groups of 3, 6 and 9 mice per cage to reflect different stocking densities. Endoparasites were examined by direct faecal smear, perianal tape test, faecal floatation and gastrointestinal examination techniques. Ectoparasites were identified under fur pluck method, tape impression test and carcass immersion while blood smear techniques were performed for blood parasites detection. Samples were taken weekly for a total of 5 weeks. Results revealed infection with pinworms; Syphacia obvelata and Aspiculuris tetraptera. Statistical analysis revealed no association between parasites and various stocking density using repeated-measures ANOVA. However, gastrointestinal examination mentioned as the ‘gold standard’ revealed an association using one-way ANOVA when P < 0.05. Overall, the results varied according to the parasitological methods used and stocking densities do not play a role in the parasitic levels of laboratory mice.
Keywords | Aspiculuris tetraptera, Laboratory mice, Pinworms, Stocking density, Syphacia oblevata
Received | April 17, 2020; Accepted | July 15, 2020; Published | August 10, 2020
*Correspondence | Saulol Hamid Nur-Fazila, Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia; Email: nurfazila@upm.edu.my
Citation | Ain-Fatin R, Nur-Fazila SH, Nur-Mahiza MI, Yasmin AR, Goh YM (2020). Stocking density associated with parasitic infection by gastrointestinal examination in laboratory mice. Adv. Anim. Vet. Sci. 8(10): 1063-1067.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.10.1063.1067
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ain-Fatin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
One of the most commonly utilized animal models in research is the laboratory mice (Mus musculus) but they are seldom investigated for parasitic infection prior to selection in use for testing. Conventional animals may be infected by ectoparasites and endoparasites that can influence the interpretation of results if the parasites are undetected and exist in high burden (Baker, 2007; Pritchett, 2007).
Notifiable ectoparasites and endoparasites have been documented in the laboratory rodents (Medeiros, 2012). Pinworms of the family Oxyurids are helminths of major importance to the laboratory mice; Syphacia obvelata (S. obvelata) and Aspiculuris tetraptera (A. tetraptera). The pinworms can be differentiated by morphological differences of the ova and adult worms (Baker, 2007; Pritchett, 2007; Taffs, 1976). The most common ectoparasites found in the laboratory mice are fur mites; Myocoptes musculinis (M. musculinis) and Myobia musculi (M. musculi). Blood parasites are rarely reported in laboratory rodents but mentionable species include the Plasmodium spp., Hepatozoon spp., and Haemabartonella spp. (Sirois, 2005).
Recommendation of cage sizes is made based on the animals’ weight and stocking density (Gonder and Laber, 2007). However, this might not be the scenario in real-life instances especially in animal facilities with limited spaces. Due to limited documentation on the effects of different management factors implemented at conventional animal facilities on the parasitic infection of laboratory animals, the objective of our study is to assess the level of parasitic infection of different stocking densities in BALB/c mice. Obtaining laboratory animals from a reliable source is imperative when its intended use is for research and development. Thus, appropriate control and preventive measures of the transmission of diseases can be made earlier before the animals are used for research purposes.
MATERIALS and METHODS
All protocols described were undertaken in accordance with criteria approved by the Universiti Putra Malaysia (UPM) Institutional Animal Care and Use Committee (IACUC) with the approval code of UPM/IACUC/AUP-R087/2018. Fifty-four (54) adult male BALB/c age 4 to 5 weeks were randomly chosen from one coventionally-maintained animal house located in Klang Valley, Malaysia. The mice were placed in groups of 3, 6 and 9 mice per cage throughout the 5 weeks-span. Three replicates of each group were done throughout the study. A group of 6 mice served as controls. They were maintained in an indoor conventional facility within open-top cages containing wood bedding. Food and drink were provided ad libitum. Cages and bedding were changed twice-weekly in a dedicated biosafety cabinet. The room was maintained on a 12 hours light:dark cycle, temperature of 18 ± 2°C and relative humidity of 30% to 50%. Management of the experimental animals were consistent with exisiting practices in the animal facility. All procedures were consistent with the Guide for the Care and Use of Laboratory Animals (NRC, 2011).
Detection of endoparasites was performed using perianal tape test, direct faecal smear, faecal floatation and direct examination of gastrointestinal tract contents. Ectoparasites were examined using tape impression, fur pluck and carcass immersion. Blood parasites were detected by thin and thick blood smear tests. The parasitological methods are in accordance with studies by Parkinson et al. (2011). The morphology of the endoparasites were identified using a compound microscope at magnifications of 10× and 40× objectives. Identification of the parasites were made corresponding to studies by Chan (1952) and Pritchett (2007).
Statistical analysis was carried out using the software Statistical Packages for the Social Sciences (SPSS) version 24. Multivariate analysis of variance (MANOVA) of repeated measures was used for parasitological methods bound towards the 5-weeks sampling to assess parasitic infection of different stocking densities in BALB/c mice. One-way analysis of variance (ANOVA) was tested for techniques performed once to assess the level of parasitic infection of different stocking densities. Data were considered as significant when P ≤ 0.05.
RESULTS AND DISCUSSION
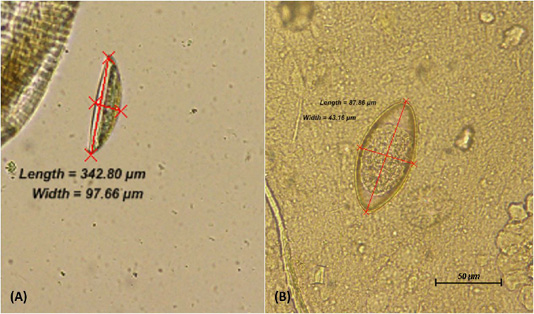
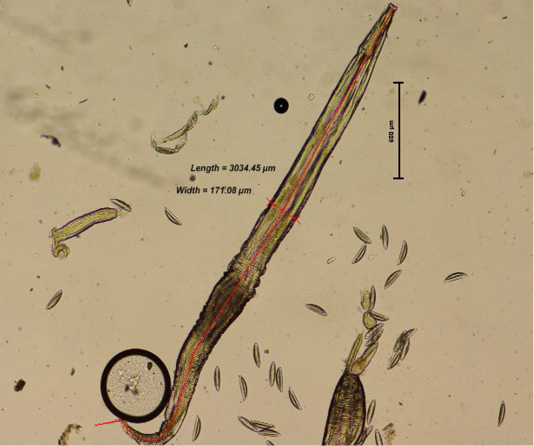
Comparison between the three groups; Group 1 (3 mice per cage), Group 2 (6 mice per cage) and Group 3 (9 mice per cage) was performed. From direct faecal smear technique, the adult worm and ova recognised in the infected mice were from the species of Syphacia obvelata (S. obvelata) and Aspiculuris tetraptera (A. tetraptera.). Identification of the helminths were made based on distinct characteristics of anterior and posterior anatomical structure as well as the ova morphology. Using the perianal tape test, the S. obvelata ova was primarily observed. S. obvelata ova could be recognized as pointed ovals measuring an average of 134 x 36 µm that are flattened on one side (Chan, 1952; Pritchett, 2007) while A. tetraptera ova recognized by faecal floatation are ellipsoidal and symmetrical in shape with an average size of 86 x 37 µm (Pritchett, 2007) as seen in Figure 1. The adult S. obvelata can be differentiated by its subtle cervical alae, round oesophageal bulb, and pointed tail as observed in Figure 2.
This particular study revealed that the laboratory mice are infected with low burden of helminths of the family Oxyuridae; S. obvelata and A. tetraptera. Generally, the mice were infested with the helminths at low levels with lesser than 300 eggs count for S. obvelata and not more than 10 eggs count for A. tetraptera. This is considered low as the S. obvelata female deposits an average of 350 eggs (Chan, 1952) whereas, the A. tetraptera female releases an average of 17 eggs daily (Phillipson, 1974). According to Bazzano et al. (2002), the prevalence of the pinworms in laboratory mice can range between 9 to 74% for S. obvelata and range between 17 to 83% for A. tetraptera. The common presence
Table 1: Relationship between stocking density and parasitic levels in BALB/c mice using various parasitological methods by repeated measures ANOVA.
| Source | Test | Sum of square (ss) | df | MS | F | P-value (Sig.) |
| Direct faecal smear | ||||||
| Between-subject effects group | 0.400 | 2 | 0.200 | 1.000 | 0.422 | |
| Within-subject effects | ||||||
| Time | Sphericity Assumed | 1.422 | 4 | 0.356 | 3.048 | 0.036* |
| Greenhouse-geisser | 1.422 | 1.583 | 0.898 | 3.048 | 0.102 | |
| Huynh-feldt | 1.422 | 2.774 | 0.513 | 3.048 | 0.061 | |
| Time x group | Sphericity assumed | 1.378 | 8 | 0.172 | 1.476 | 0.218 |
| Greenhouse-geisser | 1.378 | 3.167 | 0.435 | 1.476 | 0.283 | |
| Huynh-feldt | 1.378 | 5.548 | 0.248 | 1.476 | 0.248 | |
| Perianal tape test | ||||||
| Between-subject effects group | 0.007 | 2 | 0.004 | 0.031 | 0.970 | |
| Within-subject effects | ||||||
| Time | Sphericity assumed | 8.493 | 4 | 2.123 | 15.568 | 0.000*** |
| Greenhouse-geisser | 8.493 | 2.839 | 2.992 | 15.568 | 0.000*** | |
| Huynh-feldt | 8.493 | 3.141 | 2.704 | 15.568 | 0.000*** | |
| Time x group | Sphericity assumed | 0.289 | 8 | 0.036 | 0.265 | 0.976 |
| Greenhouse-geisser | .289 | 5.677 | 0.051 | 0.265 | 0.947 | |
| Huynh-feldt | .289 | 6.283 | 0.046 | 0.265 | 0.957 | |
| Faecal floatation | ||||||
| Between-subject effects group | 0.844 | 2 | 0.422 | 2.111 | 0.202 | |
| Within-subject effects | ||||||
| Time | Sphericity assumed | 2.800 | 4 | .700 | 4.846 | 0.005** |
| Greenhouse-geisser | 2.800 | 2.139 | 1.309 | 4.846 | 0.025* | |
| Huynh-feldt | 2.800 | 4.000 | .700 | 4.846 | 0.005** | |
| Time x group | Sphericity assumed | .933 | 8 | .117 | .808 | 0.602 |
| Greenhouse-geisser | .933 | 4.278 | .218 | .808 | 0.549 | |
| Huynh-feldt | .933 | 8.000 | .117 | .808 | 0.602 | |
*P<0.05, **P<0.01, ***P<0.005: considered to be significantly different. df, degree of freedom; MS, mean square; F, ratio of two variances.
of the pinworms are attributed by the difficulty to achieve complete eradication as the usage of anthelminthic can only get rid the adult worms but do not affect the ova which can survive for long period of time (Baker, 2007).
The association between parasitic infection and different stocking densities are evaluated in Table 1. No significant differences were obtained between different stocking densities towards endoparasites infection using methods of direct faecal smear, perianal tape test, and faecal floatation. Meanwhile, gastrointestinal examination method revealed gravid S. obvelata female worms without any presence of A. tetraptera worm. It was found that the group with the highest stocking density of 9 mice per cage had the most number of adult worms found with a total of 82 worms with the highest presence of adult worms by 83.33% (n= 5; N= 6). Both group 1 and controls had results of 50% (n= 3; N= 6) for presence of worms, but the amounts of worms found in both groups are considerably distant. Statistically, this method demonstrated a significant difference between stocking densities and endoparasites infection (Table 2). Other than that, ectoparasites and blood parasites were absent for all groups throughout the study.
Our study showed that parasitic infections were uncorrelated with different stocking densities using different parasitological methods within a 5-week period except gastrointestinal examination. A previous study has recommended a floor area based on the animal’s weight and the number of animals in a cage (Gonder and Laber, 2007). As a guide, the ideal number of mice per group is three to five for females and three for males. Meanwhile, other study reported that increase group size exhibited higher aggression and physiological disturbances (Van Loo et al., 2001). Thus, this study aimed to observe whether different stocking densities affect the infection of parasites in the laboratory mice. Although our results showed otherwise, we cannot rule out the possibility of individual age related resistance in mice associated to an increase of mucus production (Taffs, 1976) causing reduction in parasitic infection with age or time.
Table 2: Relationship between stocking density and parasitic levels in BALB/c mice using gastrointestinal examination by one-way ANOVA method.
| Presence of worms | |||||
| ANOVA | Sum of squares (ss) | df | MS | F | P-value (Sig.) |
| Between-groups | 3760.774 | 2 | 1880.387 | 23.487 | 0.000*** |
| Within-groups | 11689.051 | 146 | 80.062 | ||
| Total | 15449.825 | 148 | |||
***P<0.005: considered to be significantly different. df, degree of freedom; MS, mean square; F, ratio of two variances.
The current study also demonstrates the advantage of gastrointestinal examination for detection of pinworms. The prevalence of S. obvelata ranged from 50% to 83% between the three groups. Statistical analysis revealed an association between stocking density and the parasitic levels by this technique. Direct examination of the gastrointestinal contents has been described as the ‘gold standard’ for pinworm detection (Dole et al., 2011). It was observed in our results where some mice found to be positive using the gastrointestinal examination although other methods revealed otherwise. However, only gravid S. obvelata females were seen by this technique. This is in agreement to a study (Bazzano et al., 2002) which revealed a higher frequency of S. obvelata detection as compared to A. tetraptera. It can be attributed by Syphacia’s shorter life-cycle that induces the infection in a larger number of mice in short periods (Baker, 2007). The presence of only female S. obvelata can be explained by the male’s shorter life span as they usually die after mating and thus, are rarely recovered (Khalil et al., 2014).
It is imperative to ensure the reliability of the source when obtaining laboratory mice to be used as animal model for any research study. Previous studies have shown that infected animals are unsuitable for any critical work because nutritional and blood values may be affected by the parasitic infestation (Griffiths, 1971). Thus, ensuring animals intended for research are ‘microbiologically’ clean should be the utmost importance. Overall, it was discovered that results vary according to parasitological methods used and no association between parasitic infection and stocking densities except by gastrointestinal examination technique.
ACKNOWLEDGEMENTS
The authors wish to thank Universiti Putra Malaysia (UPM) as a research grant provider (GP-IPM/2017/9526900) and also to all the staff of Animal Resource Unit and Veterinary Parasitology Laboratory, Faculty of Veterinary Medicine, UPM who helped in the project particularly, Mrs. Maizatul, Mr. Rashid, Mr. Zainuddin, Mr. Aizat and Mr. Ismail Shaari.
AUTHORS CONTRIBUTION
SHNF designed the study, and RAF carried out the experiment, sample collection, data analysis, and drafted the manuscript. SHNF, ARY and MINM were critically revised the manuscript. YMG was involved in data analysis and interpretation. All the authors have read and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declared that there is no conflict of interest.
REFERENCES