Advances in Animal and Veterinary Sciences
Case Report
Trypanosoma vivax Epizootic Infection in Cattle from Espírito Santo State, Brazil
Rabeche Schmith1, Winny Silva Moreira1, Jeferson Miguel Vassoler1, Júlio Cesar Vassoler1, José Eduardo Guimarães de Britto Souza1, Alexandre Granados Afonso de Faria1, Leonardo Campos Almeida1, Mariana Caetano Teixeira2, Clairton Marcolongo-Pereira1*
1Faculty of Veterinary Medicine, Centro Universitário do Espírito Santo - UNESC, Colatina, Brazil; ²Faculty of Veterinary Medicine, Centro Universitário Ritter dos Reis–UniRitter, Porto Alegre, Brazil.
Abstract | Bovine trypanosomiasis is a parasitic disease caused by the protozoan Trypanosoma vivax. This disease causes high morbidity and mortality in the herd and is thus responsible for low economic savings in cattle farming. The objective of this study was to report the occurrence of trypanosomiasis due to T. vivax in Espírito Santo, Brazil. The animals had reduced milk production, intermittent fever, anorexia, apathy, births of weak cattle, and adult mortality after progressive weight loss. Complete blood count and hematozoa were investigated. In the hematological analysis, the trypomastigote form of T. vivax was observed. This was the first report of the occurrence of T. vivax in the State of Espírito Santo. The results of this paper show the importance of carrying out studies to diagnose the epidemiological situation of the disease within the productive context of regions that manage dairy farming with severe symptoms of the disease. Therefore, the occurrence of trypanosomiasis can have a negative impact on the health and economics of local cattle farming.
Keywords | Bovine, Trypanosoma vivax, Parasite, Infection
Received | March 13, 2020; Accepted | May 19, 2020; Published | May 25, 2020
*Correspondence | Clairton Marcolongo-Pereira, Faculty of Veterinary Medicine, Centro Universitário do Espírito Santo–UNESC, Rua Fioravante Rossi 2930, Bairro Martinelli, Colatina-ES, Brazil, 29703-858; Email: clairton.marcolongo@terra.com.br
Citation | Schmith R, Moreira WS, Vassoler JM, Vassoler JC, Souza JEGB, Faria AGA, Almeida LC, Teixeira MC, Marcolongo-Pereira C (2020). Trypanosoma vivax epizootic infection in cattle from Espírito Santo State, Brazil. Adv. Anim. Vet. Sci. 8(6): 629-632.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.629.632
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Schmith et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine trypanosomiasis is a parasitic disease caused by the protozoan Trypanosoma vivax. This disease causes high morbidity and mortality in the herd, and is thus responsible for significant economic losses in cattle (Lopes et al., 2018). The transmission of the disease-causing agent occurs mainly through the blood meal of blood-sucking insects (Vieira et al., 2017). In African countries, where the disease is widely distributed, the main biological vector is the tsetse fly, whereas in the Americas, the flies: Stomoxys calcitrans and Tabanus spp. predominate as vectors (Reis et al., 2019). In addition, transmission can occur via iatrogenic means: application of drugs, blood transfusions, and fomites (Vieira et al., 2017).
Animals infected with the parasite can present the following forms: acute, chronic, or asymptomatic, and the main clinical signs are characterized by reduced milk production, acute anemia, anorexia with progressive weight loss, hyperthermia, reproductive problems including abortions, and neurological signs (Vieira et al., 2017; Pereira et al., 2018).
The diagnosis of the disease can be made by a combination of assessment of the clinical picture and parasitological, serological, and molecular techniques. In general, the diagnostic methods used to identify T. vivax are the detection of the hemoparasite by means of direct blood smear, serological assays such as indirect immunofluorescence assay (IFA) (Guerra et al., 2013), enzyme-linked immunosorbent assay (ELISA) (Madruga et al., 2006), and molecular detection by polymerase chain reaction (PCR) (Vieira et al., 2017).
In recent years, there has been a greater record of outbreaks of bovine trypanosomiasis in Brazil (Barbosa et al., 2015; Pereira et al., 2018). The present study aimed to report the occurrence and risk factors associated with T. vivax infection in cattle in the State of Espírito Santo, Brazil.
CASE DESCRIPTION
The study was carried out in 2019 on a dairy property located in the municipality of Aracruz, Espírito Santo. The farm herd consisted of Girolando cows, with a total production of 360 liters per day and an average of 16.36 kg milk per cow per day. The production system adopted was intensive rotated grazing, with two mechanized milking daily.
There were 22 cows on the property, 10 of which had clinical signs of the disease, characterized by Decreased milk production, intermittent fever, anorexia, apathy, birth of weak calves, and mortality of adult animals after progressive weight loss, were reported by the owner. For diagnostic confirmation, whole blood samples were collected from the symptomatic cattle via jugular venipuncture. The collected samples were stored in 4-mL sterile tubes with EDTA anticoagulant.
Complete blood count was performed using an automated hematology analyzer (URIT-3000 Vet Plus – MHLab®), calibrated to cattle as recommended by the manufacturer. Slides for protozoan investigation and differential blood count were prepared. The slides were fixed in methanol and stained using Giemsa, and were examined under immersion on optical microscope. In the hematological examinations, the alterations observed were normochromic normocytic anemia, thrombocytopenia, and leukocytosis in all animals. In the slides analysis, the trypomastigote form of T. vivax was observed (Figure 1).
All cattle in the herd, after identifying the parasite in the blood samples, were treated with intramuscular isometamidium hydrochloride, at a dose of 1.0 mg/kg. Forty days later, the animals received a new dose of the drug, at a dose of 0.5 mg/kg.
Fifteen days later, a new blood test to check for hematozoa on the previously positive animals as well as molecular detection by PCR were carried out.
Genomic DNA was extracted from 200 µl of bovine blood using a commercial kit (Qiagen DNeasy blood and tissue kit; Hilden, Germany) following the manufacturer’s recommendations. PCR was performed as described by Ventura el at. (2001) and modified by Cuglovici et al. (2010), using the primers 18S TviSL1 and TviSL2, which amplified a fragment of 210 bp.
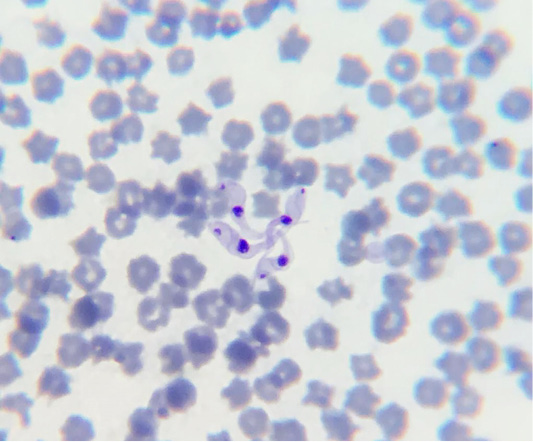
Figure 1: Trypomastigote forms of T. vivax with rounded posterior end, a weakly developed undulating membrane, central nucleus and terminal kinetoplast.
T. vivax was not observed in blood samples taken after treatment, nor were fragments of parasite DNA amplified in the PCR.
Trypanosoma vivax was not observed in blood samples taken after treatment, nor were fragments of parasite DNA amplif
DISCUSSION
The diagnosis of trypanosomiasis was based on assessment of clinical and epidemiological characteristics, and observation of the trypomastigote forms of the parasite in blood smears. These forms observed had a rounded posterior end, a weakly developed undulating membrane, central nucleus and terminal kinetoplast, characteristic of T. vivax (Hoare, 1972). Trypanosoma vivax has been mentioned as the main species that affects cattle in Brazil (Reis et al., 2019). The diagnosis of infection by this species of parasite is made on the basis of identification of the parasite’s morphology, characterized by a sickle shape, with an obtuse posterior end, a wavy membrane, a large central nucleus, a terminal kinetoplast, and a free flagellum (Lopes et al., 2018; Reis et al., 2019).
It is noteworthy that the cattle on the property began to fall ill soon after animals from other regions of Brazil, mainly from the State of Minas Gerais, were introduced into the property. It was suggested that the protozoan was introduced through the acquisition of animals with subclinical infection, acquired without examination and prior knowledge of their health status. The movement and commercialization of animals in the absence of adequate sanitary control is one of the most important risk factors for the occurrence of outbreaks of the disease as well as for its spread (Barbosa et al., 2015). In addition, the incidence of the disease probably increased within the property, through the use of shared needles, especially during the injection of the hormone, oxytocin, which is used to help eject milk. Barbosa et al. (2015) reported the relationship between outbreaks in Goiás, and the daily administration of oxytocin in cows, intravenously, before milking, using the same needle and syringe.
Another factor that may have contributed to the expansion of cases on the property was the presence of Stomoxys calcitrans. The blood meal of this hematophagous insect plays an important role in the transmission of T. vivax. in Brazil. Cadioli et al. (2012) reported Stomoxys calcitrans as the main epidemiological factor for the occurrence of the disease in an outbreak, in dairy cattle, in the State of São Paulo, while Lopes et al. (2018) associated the increase in cases of Trypanosomiasis to the presence of Tabanus spp. and S. calcitrans in Parnaíba, Piauí.
In this study, the animals showed marked anemia. Anemia is described as one of the main findings of trypanosomiasis, which may be a consequence of some pathological events such as decreased erythropoiesis, vascular hemolysis, erythrophagocytosis and inadequate hematopoiesis (Batista et al., 2008; Lopes et al., 2018; Pereira et al., 2018).
Disease control can be achieved through drugs such as diminazene acetate and isometamidium hydrochloride (Cadioli et al., 2012). However, in order to effectively control the disease, it is necessary, in addition to treating the affected animals, to restrict the movement of sick cattle, monitor the distribution of the disease, and control the vectors (Mbewe et al., 2015). After treating the animals with isometamidium hydrochloride, the blood smear and the PCR result for T. vivax was negative, as observed in another study (Reis et al., 2019).
CONCLUSION
This was the first report of the occurrence of T. vivax in the State of Espírito Santo. The results of this study show the importance of carrying out new studies to determine the epidemiological situation of the disease within the productive context of regions that manage dairy farming with severe symptoms of the disease. Therefore, the occurrence of trypanosomiasis can have a negative impact on the health and economics of local cattle farming.
ACKNOWLEDGEMENT
To Fundação de Amparo à Pesquisa e Inovação do Estado do Espírito Santo - FAPES for granting the necessary financial support for the development of this study.
Authors Contribution
All authors were actively involved in the study and approved the final draft of this manuscript.
CONFLICT OF INTEREST
The authors have no competing interests.
REFERENCES






