Advances in Animal and Veterinary Sciences
Research Article
In vitro Assessment of Antimicrobial Activity of Zinc Oxide Nanoparticles against Pathogenic Streptococcus parauberis
Fatma A. Fadl1*, Nashwa A. Abu aita1, Mohamed A. Abdelaziz2, Amira H. Mohamed1
1Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; 2Department of Aquatic Animal Medicine and Management, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt.
Abstract | Antibiotic resistance is a global issue that threatens public health. Searching for an eco-friendly antimicrobial agent that shows a positive effect against antibiotic-resistant pathogenic microorganisms is highly recommended. Nano size inorganic compounds have been shown a remarkable antimicrobial activity. The current study aims to assess the in vitro antimicrobial activity of chemically synthesized ZnO nanoparticles (ZnO NPs) against an isolated pathogenic strain of Streptococcus parauberis from an infected fish farm in Ismailia governorate, Egypt. Well diffusion test was performed to provide a qualitative assessment of the antimicrobial activity along with the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the tested NPs. Additionally, the effect of NPs on the bacterial cell was investigated via viability test through 2, 3, 5-triphenyl-2H-tetrazolium chloride (TTC) and transmission electron microscopy (TEM) imaging. ZnO NPs showed clear inhibition of the visible bacterial growth at 0.125 mg/ml. and 0.250 mg/ml. as shown by MIC and MBC respectively. The bacterial cell viability was significantly reduced via adherence of the NPs to the cell membranes, and disrupting the membrane integrity along with leakage of the cellular protein that was validated by the protein Bradford assay. Collectively, these findings provide an essential basis for the development of a new strategy that opens a new avenue to curb fish streptococcal infections.
Keywords | Antibiotic resistance; ZnO nanoparticles; MIC; S. parauberis; TTC.
Received | March 12, 2021; Accepted | March 17, 2021; Published | May 25, 2021
*Correspondence | Fatma A Fadl, Department of Clinical Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; Email: fatmafadl@cu.edu.eg
Citation | Fadl FA, Atta NAA, Abdelaziz MA, Mohamed AH (2021). In vitro assessment of antimicrobial activity of zinc oxide nanoparticles against pathogenic streptococcus parauberis. Adv. Anim. Vet. Sci. 9(6): 913-918.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.6.913.918
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Fadl et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Streptococcal infection is a major bacterial problem that affects a wide variety of fish species, causing heavy economic losses in the aquatic industry in multiple countries all over the world (Baeck et al., 2006; Cai et al., 2016). Streptococcosis is caused by various streptococcal species (Lazado et al., 2017), chief among them, Streptococcus parauberis (S. parauberis). It is a non-motile, α- hemolytic, gram-positive cocci belongs to family Streptococcacea (Nho et al., 2011). Streptococcus parauberis has been incriminated to cause severe diseases in turbot (Scophthalmus maximus), striped bass (Morone saxatilis), sea bass (Sebastes ventricosus), and olive flounder (Paralichthys olivaceus) (Domeenech et al., 1996; Mata et al., 2004; Baeck et al., 2006; Park et al., 2009; Oguro et al., 2014). Furthermore, serious public health problems and zoonotic potential from some streptococcal species including S. parauberis have been reported (Ghittino et al., 2003; Zaman et al., 2016).
Streptococcosis control measures depend primarily on two pillars; vaccinations and antimicrobial compounds (Darwish and Hobbs 2005; Håstein et al., 2005; Cheng et al., 2010). However, molecular-based approaches for vaccinations have been described for S. parauberis, the majority of commercial vaccines have been confronted with limited success in the application phase (Woo and Park, 2014). Vaccination failure is also common in the aquatic industry due to the diversity of fish species, fish size, vaccine formulation, and the route of administration (Toranzo et al., 2005). Moreover, serotypic variations in some streptococcal species are also incriminated in vaccination failure (Agnew and Barnes, 2007). On the other hand, the extensive and unsystematic use of antibiotics in fisheries lead to the development of drug-resistant strains (Park et al., 2009).
Recently, nanotechnology is considered an emerging discipline of sciences that deals with the synthesis and application of materials less than 100 nm in size. It is widely used in several indispensable fields of life; agriculture, food industry, medical, and diagnostic applications (Siddiqi et al., 2018). The mechanism of action of nanoparticles has been elucidated to their high surface area to volume ratio and their unique chemical and physical features (Rai et al., 2009; Siddiqi et al., 2018). Moreover, these compounds are more stable at different temperatures and pressures (Sawai 2003). Among these nanoparticles, zinc oxide (ZnO) nanoparticles that considered a nontoxic vital mineral element (Roselli et al., 2003). ZnO particles have also been exhibited in notable antimicrobial activities against a diverse range of bacteria and spores (Sirelkhatim et al., 2015; Raghunath and Perumal, 2017). The current study aims to assess the potential antimicrobial activity of commercially available chemically synthesized ZnO NPs against an isolated pathogenic strain of S. parauberis that exhibited great losses in an Egyptian fish farm. The outcome of this study may help to find an alternative approach to control some bacterial infections in fish farms and open a new avenue to curb antibiotic resistance to bacterial infections.
Materials and Methods
Microbial isolate, media, and growth conditions
Streptococcus parauberis isolated from Oreochromis niloticus in a farm located in Ismailia governorate, Egypt was used in the present study. Under aseptic conditions, Swabs from the kidneys and the brain of the infected fish showing darkness of the skin, hemorrhage and skin ulcers were collected and streaked on blood agar. The isolated bacteria appeared as pinpointed colonies on the cultured agar. The bacterial isolate was then confirmed using biochemical and molecular characterization through 16s rRNA sequencing. The sequence was deposited in GenBank with the accession number MW534372 and it was identified as Streptococcus parauberis. Glycerol stocks of the strain were prepared and preserved at −80°C. The bacterial cultures maintained on trypticase soy agar (TSA) (Oxide, UK) or Müller Hinton agar (MHA) (Sigma-Aldrich, USA). Single colony inoculates were suspended in normal saline with shaking at 37°C. Mid-log bacterial suspension (1.5 × 108 CFU/ml) that equivalent to 0.5 McFarland standard were used in all experiments as described by Nazoori and Kariminik, (2018)
Nanoparticles
Chemically synthesized ZnO NPs of average size 30 ± 5 nm were purchased from Nanotech center, 6th October, Giza, Egypt. Nanoparticle suspension was prepared by dissolving nanoparticles powder (1mg/ml) in deionized water as described by Emami-Karvani and Chehrazi (2011).
Evaluation of Antibacterial Activity of ZnO NPs
Qualitative assessment of the synthesized ZnO NPs antimicrobial activity was performed using the well diffusion test as previously described by El-borady et al. (2018). An antibiotic sensitivity test was conducted at first to determine the antibiotic of choice that will be used for well diffusion test. Ampicillin, amoxicillin, and erythromycin (Sigma-Aldrich, UK) were used for sensitivity test. S. parauberis bacterial suspension was cultured on MHA plates. Several wells were generated in agar plates using sterile metal circles. ZnO NPs, ampicillin (Sigma-Aldrich, UK) (served as the strongest positive control), and normal saline (served as a negative control) were added separately to the formed wells. All cultures were incubated at 37ºC for 24 hours. The diameter of inhibition zones was measured and recorded. All values were expressed as the average of three independent experiments.
Determination of MIC and MBC
Minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) were carried out to quantitatively determine the antibacterial activity of the synthesized product. To determine the lowest concentration that can inhibit the visible growth of bacteria completely, S. parauberis was treated with decreasing concentrations of ZnO NPs for 24 h at 28ºC. MIC was performed by macro- and micro-dilution techniques according to Balouiri et al. (2016). For macro-dilution technique, sterile screw-capped tubes with 2 ml Müller Hinton Broth (MHB) containing different concentrations of ZnO NPs (1000, 500, 250, 125, 62.5 and 31.25 µg/ml) were inoculated with 0.2 ml of 1.5 × 108 CFU/ml bacteria. A culture control tube containing 2 ml MHB inoculated with 0.2 ml of 1.5 × 108 CFU/ml bacteria was performed. Meanwhile, MHB alone was served as a negative control. All tubes were incubated at 28ºC for 24 hrs. The test was performed in triplicate. Regarding the microdilution technique, the same concentrations and controls were performed in 12 well microtiter plates. The plates were incubated at 28ºC for 24 hrs. To determine the MBC, subcultures of samples from tubes of MIC were plated on antibiotic-free TSA for 24 hrs. The lowest concentration at which almost the final inoculum is killed was determined, as described by Alekish et al. (2018).
Validation of Cell Viability (TTC Assay)
Bacterial cell viability was investigated by adding 2, 3, 5-triphenyl-2H-tetrazolium chloride (TTC) solution (Research Lab, India) to the MIC microtiter plates which were incubated for an additional three hours at 28ºC. TTC solution is a colorless solution that is reduced to red formazan in the presence of viable cells. The intensity of red formazan (OD) is directly proportional to the predominant metabolically active cells. The absorbance was measured at 570 nm. Viability percentage was calculated as the ratio between the absorbance of treated (ZnO-NP-containing wells) versus the absorbance of culture control. All values were expressed as the average of three independent experiments (Tiwari et al., 2018) .
Testing the antimicrobial action of ZnO NPs using TEM
Streptococcus parauberis was cultured in the presence and absence of ZnO NPs. Samples of macro-dilution technique were centrifuged at 3000×g for 15 min. to get rid of the supernatants. The bacterial precipitate was collected for transmission electron microscopy (JEOL, USA) at the TEM unit, Ain Shams University, Egypt, according to Abdel-Razek (2019).
Quantification of Membrane Leakage of cellular Proteins
The effect of ZnO NPs on membrane leakage of proteins released from the intracellular cytosol of the bacterial cells after treatment with ZnO NPs was estimated. MHB broth was treated with ZnO NPs (1 mg/ ml) and overnight incubated at 37 °C, the culture centrifuged at 10,000g for 30 min at 4°C. Bradford method was used for protein quantification at 595 nm according to Bradford (1976).
Statistical analysis
All statistical analyses were conducted using the R software package (ver. 3.6.1) (R Core Team, 2019). Results were obtained as a mean ± 95% confidence interval (CI) for the mean (Independent t-test). The significance level was set at a probability value of less than 0.05 (p ˂ 0.05).
Results
Antibacterial Activity of ZnO NPs (Well Diffusion Assay)
The antimicrobial activity of ZnO NPs was seen in the form of a clear zone around the ZnO NPs well (Fig. 1a). The diameter of the inhibition zone was recorded in (Table 1). Statistical analysis of the present data revealed non-significant difference between the ZnO NPs inhibition zone compared with ampicillin.
Results of MIC and MBC
ZnO NPs inhibited the visible bacterial growth in a dose-dependent manner. The lowest concentration inhibited the visible growth of S. parauberis (MIC) was observed at 0.125 mg/ml (Fig. 1b). Meanwhile, the lowest concentration of ZnO NPs that prevented the growth of the bacteria on antibiotic-free TSA culture media (MBC) was 0.250 mg/ ml as illustrated in (Fig. 1c).
TTC Assay Result
Percentage of viable cells of culture control and ZnO NPs treated groups were recorded in Table (2). Statistical analysis of the obtained data revealed marked decrease in the cellular viability of ZnO NPs treated bacterial culture (Fig. 1d).
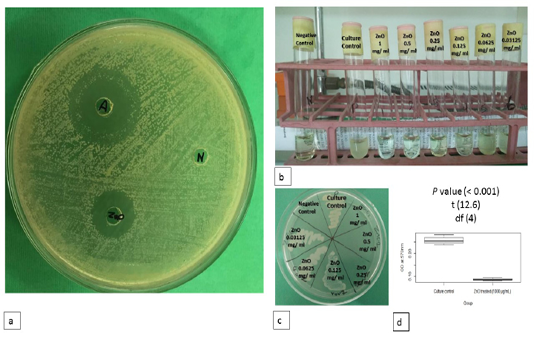
Figure 1: Antimicrobial activity of ZnO NPs. a) Antimicrobial activity of ZnO NPs on MHA. b) MIC of ZnO NPs against S. parauberis on MHB. c) MBC of ZnO NPs against S. parauberis on antibiotic free TSA. d) Cell viability after treatment with ZnO NPs using TTC assay. Positive and negative controls are indicated.
Table 1: Diameter of inhibition zone of Ampicillin and ZnO NPs against S. parauberis by well diffusion test.
| Groups | Diameter of inhibition zone (mm) |
| Ampicillin |
27.00 ± 1.96 a |
| ZnO NPs |
26.67 ± 3.45 a |
Values represent mean ± CI 95.
Table 2: Effect of ZnO NPs on S. parauberis viability %
| Groups | OD | Viability % |
| Culture control |
0.258 ± 0.025 a |
100 ± 0.00 a |
| ZnO treated |
0.086 ± 0.006 b |
33.60 ± 3.62 b |
Values represent mean ± CI 95.
Transmission Electron microscopy results
ZnO NPs treated bacterial culture
revealed attachment of the NPs to the bacterial cell membranes, and NPs were also noticed in the cell cytoplasm with concomitant rupture of the cell membrane and release of intracellular contents. Some bacterial cells showed empty regions in the cytoplasm. Transmission electron microscopy of bacteria cultured in absence of ZnO NPs appeared normal (Figure 2).
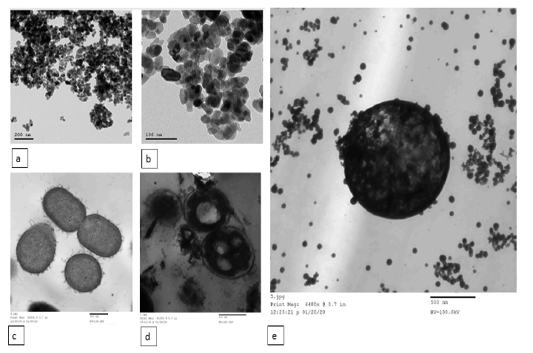
Figure 2: Transmission electron microscopy (TEM) of ZnO NPs. (a and b) the shape of the synthesized ZnO NPs under TEM. TEM image of S. parauberis cultured in the absence (c) and presence (d) of chemically synthesized ZnO NPs. e) ZnO NPs attached to the bacterial cell membrane and appeared intracellularly. The scale bars are indicated.
Quantification of Membrane Leakage of cellular Proteins
The effect of ZnO NPs on membrane leakage of cellular proteins was presented in Table (3). Statistical analysis of the Bradford protein results illustrated that the lleaked cellular proteins in ZnO treated culture were 2 folds higher compared to that of culture control.
Table 3: Membrane leakage of cellular proteins of S. parauberis treated with ZnO NPs
| Group | Protein (µg/ml) |
| Culture control |
11.70 ± 2.86 b |
| ZnO treated |
20.03 ± 3.86 a |
Values represent mean ± CI 95.
Discussion
The increasing concern about resistant microorganisms stimulates the study of new and more effective antimicrobial agents (Raghunath and Perumal, 2017). Good antimicrobial effects have been obtained from metal oxide nanoparticles. Zinc oxide nanoparticles (ZnO-NPs) have been reported as an antimicrobial agent against a diverse range of bacteria including Staphylococcus aureus, Escherichia coli (Alekish et al., 2018), Campylobacter jejuni (Xie et al., 2011), Salmonella typhimurium (De Souza et al., 2019) and Acinetobacter baumannii (Tiwari et al., 2018).
In the present study, ZnO NPs of average size 30 ± 5 nm were tested for their in vitro antimicrobial activity against and identified strain of S. parauberis isolated from diseased fish belong to a fish farm in Egypt. The well diffusion test was performed as a qualitative assessment of the antimicrobial activity of ZnO NPs. Meanwhile, both macro and microdilution techniques followed by sub culturing were performed to determine the MIC and MBC of the tested NPs against S. parauberis. Furthermore, the mechanism of the effect of ZnO nanoparticles was based on the assessment of the bacterial cell viability, TEM imaging, and the leaked cellular proteins.
Results of broth dilution techniques showed that ZnO NPs were capable to inhibit the visible bacterial growth at 0.125 mg/ml. The obtained results are in parallel with Liang et al. (2020) who recorded a dose dependent growth inhibition of Streptococcus pyogen bacterial suspension treated with ZnO NPs (49.9 nm). Nazoori and Kariminik (2018), reported that MIC of ZnO NPs (121-128 nm) was estimated to be more than 2.5 mg/ml against S. aureus, S. marcescens, and E. coli and was 5 mg/ml against P. aeruginosa, A. baumannii, and K. pneumoniae. The difference in MIC could be attributed to the different sizes of the nanoparticles and the tested bacterial strain.
The broth dilution assay can be considered confirmative and more accurate than the well diffusion assay as the chances of nanoparticle-bacteria interactions are higher in the liquid phase as suggested by De Souza et al. (2019).
Regarding the viability test, the optical density of S. parauberis culture control group was three-fold higher than ZnO NPs treated group which indicate that ZnO NPs were capable to significantly decrease bacterial cell viability. Our results are supported by the transmission electron microscopy as ZnO NPs treated bacterial culture showed clear adherence of ZnO NPs to the bacterial cell membranes with the presence of ZnO NPs inside the cell cytoplasm. Moreover, some bacterial cultures showed ruptured membrane. The observed changes may be referred to the alterations of membrane permeability via production of ROS by ZnO NPs resulting in membrane lipid peroxidation and leakage of cellular contents which explain the significant increase of the cellular proteins of the ZnO treated culture compared to culture control. The obtained results are in accordance with Tiwari et al. (2018) who mentioned that green synthesized zinc oxide nanoparticles possess an anti-bacterial activity against Acinetobacter baumannii through disrupting the bacterial cell membrane.
The achieved results clarify the importance of nano-sized particles as a new, promising therapeutic strategy to curb fish bacterial infections, however, detailed in vivo testing are needed to assess the therapeutic significance of this product.
Funding
This work has been financially supported by the postgraduate research funds, Faculty of Veterinary Medicine, Cairo University, Egypt.
Conflict of interest
All authors declare no conflict of interest.
Author Contributions
Conceptualization: Amira H. Mohamed, Nashwa A. Abu Aita, and Mohamed A. Abdelaziz, Formal analysis and investigation: Fatma A. Fadl, Nashwa A. Abu Aita, Mohamed A. Abdelaziz, and Amira H. Mohamed, Writing - original draft preparation: Fatma A. Fadl, Writing - review and editing: Amira H. Mohamed, Nashwa A. Abu Aita, and Mohamed A. Abdelaziz, Supervision: Amira H. Mohamed, Nashwa A. Abu Aita, and Mohamed A. Abdelaziz.
References






