Advances in Animal and Veterinary Sciences
Research Article
Accurate Diagnosis of Camel Nasal Myiasis using Specific Second Instar Larval Fraction of Cephalopina titillator Extract
Nagwa I. Toaleb*, Eman H. Abdel-Rahman
Parasitology and Animals Diseases Department, Veterinary Research Division, National Research Centre Cairo, Egypt.
Abstract | Cephalopina titillator (C. titillator) larvae are considered important nasal myiasis producing agent of camels, causing significant production losses in animals, and causing huge economic loss to the livestock industry all over the world. Myiasis is considered a zoonotic disease of global importance, some myiasis can be life-threatening for humans. The infestation was diagnosed only on post-mortem examination of the animal after slaughter. Therefore, the objective of the present research was to isolate and characterize a specific fraction extracted from C. titillator larval antigen, for the specific diagnosis of early infestation in living camels. Second larval instar extract of C. titillator showed higher diagnostic potency than first and third larval instars crude extracts. So that, an immunoaffinity larval fraction was isolated from the 2nd larval instar extract of C. titillator with the highest diagnostic potency than its crude extract and unbound fraction as proved by ELISA and western blot. The fraction has antigenic activity 87.67% of the initial activities, and with the 1719.6-fold increase in specific activity compared to its crude extract. It separated into two bands with molecular weight 52 kDa and 22 kDa by SDS-PAGE. The diagnostic potency of the isolated fraction was evaluated by indirect ELISA. The fraction recorded high specificity 96.88% and 100% sensitivity. Moreover, the fraction successfully achieved 94.12%, 100%, and 97.92 % positive predictive, negative predictive, and diagnostic efficacy values, respectively. Finally, the fraction recorded a prevalence of 65.95% of nasal myiasis in collected random blood samples of living camels by ELISA. Collectively, the current results present a promising diagnostic immunoaffinity fraction of the 2nd larval instar extract of C. titillator to detect specific antibodies of nasal myiasis in camels by ELISA.
Keywords | Cephalopina titillator- Larval antigens - Affinity Chromatography -SDS-PAGE ELISA– Western blot
Received | February 02, 2021; Accepted | February 11, 2021; Published | March 15, 2021
*Correspondence | Nagwa Ibrahim Toaleb, Parasitology and Animals Diseases Department, Veterinary Research Division, National Research Centre Cairo, Egypt; Email: nagwaibrahim26@yahoo.com
Citation | Toaleb NI, Abdel-Rahman EH (2021). Accurate diagnosis of camel nasal myiasis using specific second instar larval fraction of cephalopina titillator extract. Adv. Anim. Vet. Sci. 9(5): 674-681.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.674.681
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Toaleb and Abdel-Rahman. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Myiasis, is an expression used to describe the infestation of a live animal by fly larvae, is rarely infested human. Camel nasopharyngeal myiasis was caused by larvae of C. titillator (Oryan et al., 2008). Previous studies showed that nasal fly (C. titillator) is an obligate parasite of the Oestridea family, and is known as a common parasite that attacks only camels in Egypt and other countries (El-Hawagry et al., 2020; Mohammadpour et al., 2020). Furthermore, nasopharyngeal myiasis represents the most important problems of camels because it causes a great economic loss resulting from the presence of C. titillator larvae (Abd El- Rahman, 2010; Al-Ani and Amr, 2016). Where the adult fly lays its eggs on living tissue in the base of the camels’ nostrils and progresses to become larvae; the first, second and third instar, these larvae attached to the nasopharyngeal and paranasal mucus membrane, and feed on living tissue, they remain about 11 months in the animal, they cause extensive irritation, tissue damage, and respiratory disorders (Shakerian et al., 2011; Al-Jindeel et al.,2018). Additionally, infestations impair animals, lose their appetite that causes losses in terms of weight gain, destroy host tissues, reduced milk and meat production, low hide quality, impaired fertility and abortion, the reduction of host physiological functions result in death and consequently cause high significant economic losses to camel industry (Sazmand and Joachim, 2017; Sazmand et al., 2019).
Camels play an important role in human’s life and in the economy of many areas of the world mainly in Africa and Asia (El-Hawagry et al., 2020; Mohammadpour et al., 2020), where they are adapted physiologically to survive under harsh conditions, and they are an important source of meat and milk (FAOSTAT, 2019). Moreover, camels play a crucial role in the epidemiology of parasitic diseases, zoonotic diseases and their transmission to other livestock and human (Zhu et al., 2019; Mohammadpour et al., 2020). Most previous reports of prevalence rates of C. titillator infestation varies from one country to another, based on data from slaughter houses that identified infestation during post-mortem examination of camel heads (Abd El-Rahman, 2010; Hasanizade, 2020). The prevalence of infestation in Egypt was found 79% (Abd El-Rahman 2010) and 80% (Attia et al., 2020). And 89.02% in Iraq (Al-Jindeel et al. 2018), 87.5% in Jordan (Al-Ani and Amr, 2016), and 41% (Alahmad, 2002) in Saudi Arabia and 67.6% in the same locality and 52% in the eastern area of the Kingdom (Amin, 2005). Moreover, the infestation of C. titillator larvae, and its pathological lesions on affected tissue was examined in slaughtered camels by Kissi and Assen (2018). Furthermore, an infestation of C. titillator larvae stimulates cellular and humeral immune responses, due to their interacting with the host’s immune system during the long period of infestation (Stevens et al., 2006; Oryan et al., 2008). Therefore, the development of a rapid diagnostic assay to detect antibodies of most camel parasites in living camels is important. Where the serological diagnosis of C. titillator infestation indicated that the incidence of positive reactors was higher than the detection of larvae at post-mortem examination (Sabah, 2000). Immunodiagnosis of myiasis especially with ELISA allows easy and cost-effective diagnosis in living animals, thus it permits planning time for treatment when larvae have not yet caused economic losses (Al-Jindeel et al., 2018). Recently, two diagnostic assays; indirect-ELISA and Dot-ELISA, were evaluated for diagnosis of C. titillator infestation in camels’ sera using salivary glands antigen from third-stage larvae of C. titillator (Attia et al., 2020). The success of ELISA depends mainly on the used antigen where the isolated immunodiagnostic fractions are by far better than crude extract as previously proved in several studies with different parasites (Toaleb et al., 2011a,b; Hassan et al.,2012; Hassanain et al.,2013; Toaleb et al.,2014; Shaapan et al., 2015; Hassan et al., 2016; Toaleb et al., 2020). Moreover, previous study, in which different C. titillator larval antigens; second and third crude extracts including; digestive tubule (DTc), excretory secretary products (ESP) and salivary glands (SGc) crude extracts were evaluated to detect anti-C. titillator antibodies in camel sera using indirect ELISA (Hendawy et al., 2013). Also, Yousef et al. (2016) used the indirect ELISA against the same crude antigens (SGc, ESP and DTc) from 2nd and 3rd larval instars of C. titillator to detect antibodies in the camel mucus samples in summer and winter.
Based on the above studies, the objective of the present study is to specifically diagnose C. titillator infestation in live camels using specific antigen purified from 2nd larval instar extract where an accurate diagnosis is initial step for treatment.
Material and Method
Ethical statement
The present research was carried out as described by the institutional guidelines of the National Research Centre’s Animal Research Committee under protocol number 18/198.
Animals
Three hundred seventy-five old and young camels were used in the current study. Two hundred eighty-nine live camels and eighty-six slaughtered camels.
Samples
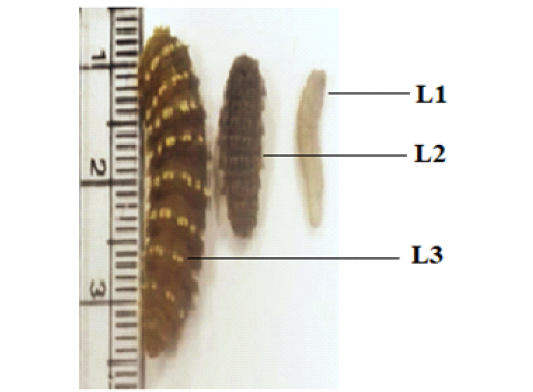
C. titillator larvae: First, second and third instars of C. titillator larvae were collected from nasal cavity, nasopharyngeal area, frontal sinuses and turbinate bones of slaughtered camels’ heads. The recovered larvae from each camel were carefully collected and were identified as C. titillator larvae according to specification of posterior spiracles as shown by Zumpt (1965). Washed the three stages; first (L1), Second (L2) and third (L3) larvae by phosphate buffer saline (PBS) PH 7.2 several times and preserved them in a separate container.
Blood samples:
Three hundred seventy-five blood samples were collected from camels. Serum samples were separated and identified as:
-Thirty-two infested camels’ sera were collected from camels infested with C. titillator larvae at post-mortem inspection, gold standard (examination the skulls of the slaughtered camels).
-Ten negative camels’ sera were collected from camels less than three months of age; proposed to be C. titillator-free, the blood samples collected in winter and before spring or summer seasons when the fly is detected. Free from C. titillator infestation and no other infection of parasites by postmortem inspection and fecal analysis (gold standard negative control sera)
-Two hundred seventy-nine randomly camels’ sera were collected from camels.
-Fifty-four blood samples from camels infected with different other parasites including; sixteen blood samples were collected from examined slaughtered camels infected with echinococcosis/hydatidosis in different organs. Seven camels’ sera were collected from slaughtered camels their liver infected with Fasciola gigantica. Twelve camels’ sera were collected from camels infested with ticks, infestation of examined dromedaries have been observed (Ticks are abundant on camels). Twelve infected camels’ sera toxoplasmosis (positive anti- Toxoplasma antibodies detection in sera of camels by ELISA). Seven infected camels’ sera with Coccidiosis (Eimeriosis) intestinal protozoan, infection of camels is caused by parasites of the genus Eimeria (proved by fecal examination).
Preparation of crude C. titillator Larval Antigens
First, second and third instars larvae were separately homogenized in phosphate buffer saline (PBS), pH 7.2 then sonicated for 5 min at pulse rate 60–80 according to Attia et al. (2019). Each homogenate was centrifuged at 16000rpm for 30min. in cooling centrifuge, and then supernatants were collected to be used as crude extracts for first, second and third larvae. The protein content of the prepared antigens was estimated by Lowry method (Lowry et al., 1951). Then the prepared antigens stored at-20°C until use.
Immunoaffinity chromatography of C. titillator 2nd instar larvae
The pooled positive naturally infected camels’ sera were coupled to the cyanogen bromide (CNBr)-activated Sepharose-4B according to manufacturer’s instructions. The crude extract of 2nd instar larval C. titillator antigen was applied to the column. Then the bound fraction was eluted and assayed for protein content according to Lowry et al. (1951).
SDS-PAGE
Under reducing condition on 10 % SDS-PAGE slab gel; the three crude extracts (First, second and third instars larvae), and isolated 2nd instar larvae fraction with stander protein were separately electrophoresed as described by Laemmli (1970). The slab gel was stained with silver stain after separation according to Wray et al. (1981). The gel was analyzed by using Gel Doc TM XR with Image Lab Software.
ELISA
Indirect ELISA was adopted firstly to evaluate the diagnostic potency of three crude extracts L1, L2 and L3 against positive C. titillator naturally infested camels’ sera. Secondly, it was selected to evaluate the success of the purification process of the most diagnostic crude extract identified in the first step, by determination of the diagnostic values of the fraction compared with the unbound one and its crude extract. Thirdly, it was performed to detect anti - C. titillator antibodies in sera of live camels using the diagnostic fraction. A checkerboard titration was used to determine the antigen, serum, and conjugate concentrations according to Harlow and Lane (1988). The reaction was read by using the ELISA reader at 450 nm (BIO-TEK, INC. ELx, 800UV). Sera which have optical density (OD) values more than the cut-off value were considered positive. The cut-off value was estimated by 2x the mean OD value of negative control sera (Romero et al., 2010).
Immunoblot analysis
After electrophoresis of the most diagnostic fraction and its crude extract, slab gel was transferred onto nitrocellulose membrane as shown by Towbin et al. (1979). The membrane was incubated with pooled antiserum of C. titillator naturally infected camel. Diluted Protein A was used. Bands were appeared by using 4-chloro-1-naphthol. The membrane was analyzed by Molecular Imager Gel Doc TM XR with Image Lab Software.
Determination of the cross reactivity and diagnostic values sensitivity, specificity, accuracy, positive predictive value (PPV), negative predictive value (NPV)
To estimate the specificity of C. Titillator diagnostic fraction, it was examined against camels’ sera infested with C. Titillator larvae, and with other parasitic Infestations using indirect ELISA. The other parasitic infections are; hard tick infestations, fasciolosis (Fasciola gigantica), echinococcosis/hydatidosis (Echinococcus granulosus), toxoplasmosis (Toxoplasma. gondii) and Coccidiosis/Eimeriosis (Eimeria spp.).
Specificity, sensitivity, positive and negative predictive values were calculated according to Tabouret et al. (2001) and Parikh et al. (2008)
Sensitivity (%) = [True positive/ (True positive + False negative)] ×100
Specificity (%) = [True negative / (True negative + False positive)] ×100
PPV % = [True positive / (True positive + False positive)] ×100
NPV % = [True negative / (False negative + True negative)] ×100.
Diagnostic Efficacy (%) = [(True negative + True positive) / (True positive +False positive + true negative + false negative)] ×100
Table 1: Quantitative summary of crude 2nd larvae purification.
|
Fraction
|
Total protein a (μg × 10 4) |
Activity unit b (Au × 106) |
Specific activity c (Au/μg × 102) |
Purification fold | Yield (%) |
| Crude ES | 424.8 | 8.2 | 1.93 | 1.00 | 100 |
| Unbound to column | 226.5 | 1.02 | .45 | .43 | 12.4 |
| Bound and eluted fraction | 32.0 | 8.0 | 25 | 1719.6 | 97.56 |
a Protein content was measured by Lowry method (Lowry et al., 1951).
b Activity unit represents the amount of protein required to give one well of agglutination.
c Specific activity, it is representing the number of activities per μg of protein and is related to the starting crude 2nd larvae
Statistical analysis
Data represented a mean OD values ± SD and analyzed using the One- way ANOVA analysis by using the SAS 9.1 software package, significance at P < .05.
Results
Larvae from the nasopharyngeal cavity of camels
Examination of 32 camel heads proved that the count of C. titillator larvae per camel was ranged between 17 and 21larvae, with a mean of 19±2 larvae per camel. The larvae were restricted to the nasopharyngeal cavity and turbinates. While a few larvae were found in the turbinate bones and ethmoid area. The colors of the first and second larvae were white or grey. The length of the first larvae ranged from 0.6 to 1.2cm (0.9 cm ± 0.3), its width was ranged from 0.2 to 0.4 cm (0.3 cm ± 0.1). Length of the second larvae was ranged from1.3 to 1.6 cm (1.45 cm ± 0.2), its width was 0.3 to 0.6 cm (0.45 cm ± 0.1). The color of the third larvae was yellowish, with a dark brown line on the ventral surface, its length ranged from 1.9 to 2.8 cm (2.35 cm ± 0.4) and its maximum width ranged from 0.6 to 1.1 cm (0.85 cm ± 0.2) as in Figure 1.
Comparative diagnostic potency of three crude Larval antigens
Crude 2nd larval antigen (L2) showed higher potency than the other crude extracts first and third larval antigens for detection of anti - C. titillator (IgG) in naturally infested camels’ sera by using indirect ELISA as shown in Figure 2.
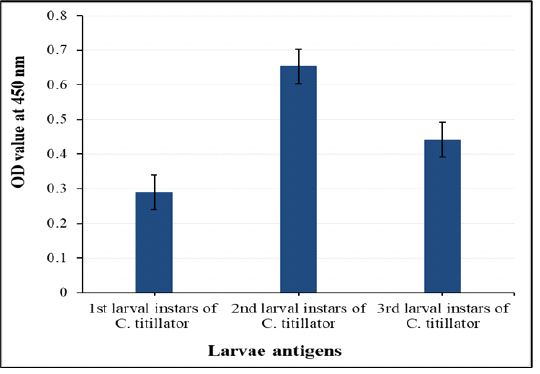
Figure 2: Comparative diagnostic potency of three crude first, second and third antigens in detection of anti- C. titillator IgG in naturally infested camels’ sera
Purified fraction of 2nd larvae antigen
Table 1 shown that the eluted fraction possesses 87.67% of the initial antigenic activities of the crude extract of 2nd larvae, and the fraction represents only 7.5% of total protein in crude 2nd larvae applied to the column giving 1719.6-fold increases in the specific activities compared to crude second larvae.
Electrophoretic profile of the antigens
The electrophoresis profile of the isolated fraction and three crude extracts; first, second and third larvae antigens under reducing conditions in SDS-PAGE 10% slab gel, showed that; isolated fraction was resolved into two bands only of molecular weight 52 kDa and 22 kDa (Figure 3 Lane 4), compared to multiple bands in its crude extract second larvae were at molecular weight 60, 52, 43, 39, 34, 29, 26, 22, 18 and 14 kDa (Figure 3 Lane 2). The predominant bands of crude extract of third larvae were eleven bands at 175, 70, 61, 53, 48, 43, 34, 29, 26, 19 and 11kDa (Figure 3 Lane 3). Whereas, crude extract of first larvae was resolved into seven bands at molecular weight 53, 43, 39, 34, 26, 24 and 10.5kDa (Figure 3 Lane 1).
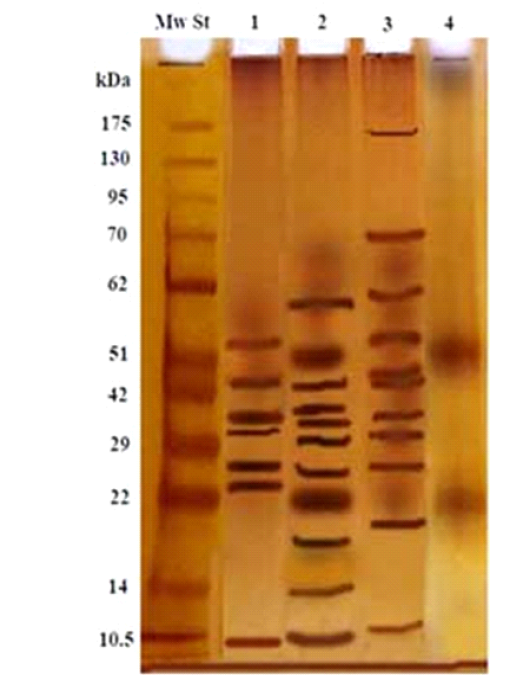
Figure 3: Electrophoretic profile of the isolated 2nd larval fraction (Lane 4), and crude first larvae (Lane 1), crude second larvae (Lane 2), crude third larvae (Lane 3) and Molecular weight standards (Lane Mw St).
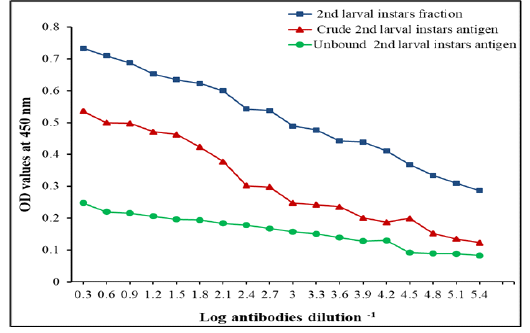
Figure 4: IgG Level measured by ELISA with specific fraction, its crude 2nd larvae and unbound antigens in detection of IgG in camels’ sera naturally infested by C. titillator larvae
Immunogenic activities of the fraction
The specific 2nd larvae fraction showed higher potency than its crude extract 2nd larvae and unbound antigens in the diagnosis of nasopharyngeal myiasis infection, in two-fold serially diluted infested camels’ sera by C. titillator larvae using indirect ELISA. The diagnostic potency of the fraction was still valid at high dilution of serum samples reached 1:262,144 (Figure 4).
Immunoblot
Immunoblot analysis used to identify the specific and sensitive immunogenic reactive bands of the fraction, using pooled sera of infested camels by Cephalopina nasal myiasis (Figure 5A) and non- infested camels’ sera negative control reacted (Figure 5B). As shown in Figure 5A four immunogenic reactive bands corresponding to molecular weight 60, 52, 22 and 14 KDa reacted specifically with infested camel sera in crude extract 2ndlarvae (Figure 5 A strip 1). Only two immunogenic reactive bands corresponding to molecular weight of 52 kDa and 22 kDa that were specific present in the fraction (Figure 5A strip 2). Both fraction and its crude extract were not reacted against control sera from non- infested camels Figure 5B (strip 3 and 4).
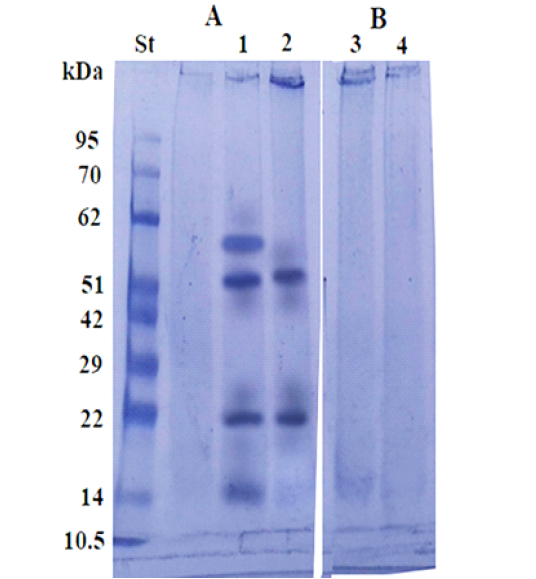
Figure 5: Immunoblot analysis of the larval purified fraction (2, 4) and its crude extract (1, 3), using pooled sera of infested (A) and non-infested camel sera by Cephalopina nasal myiasis (B).
Assessment of the antigenic activities and the immunological diagnostic values of the isolated fraction
Immunogenic reactivity of the fraction was tested by indirect ELISA to detect C. titillator antibodies levels using positive IgG antibodies of C. titillator, negative camels’ sera and different antibodies of other parasitic infections but negative for IgG of C. titillator. The fraction reacted positively with all serum samples of camel infected with C. titillatoras proved by post mortem examination, recording100% sensitivity and 96.88% specificity (Figure 6). The fraction successfully achieved 94.12%, 100% and 97.92 % positive predictive, negative predictive and diagnostic efficacy values, respectively. While the fraction recorded 8.3 % cross - reactivity (false positive) with camel serum infected with toxoplasmosis (n=12, one false positive for C. titillator nasal myiasis). No cross reactivity was recorded with uninfected camel sera (negative sera for C. titillator), and no cross reactivity with antibodies of tick’s infestation, fascioliasis, hydatidosis and coccidiosis (Figure 6).
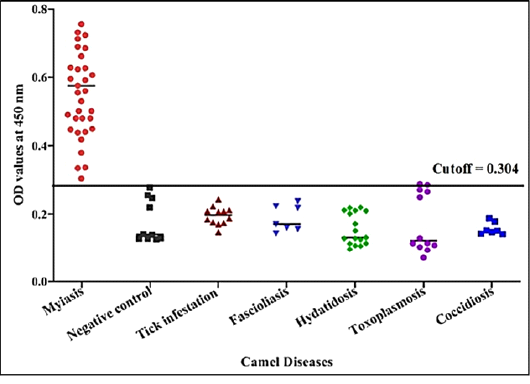
Figure 6: Detection of specific C. titillator antibodies IgG in positive camel sera and different sera of camel diseases using larval fraction by ELISA
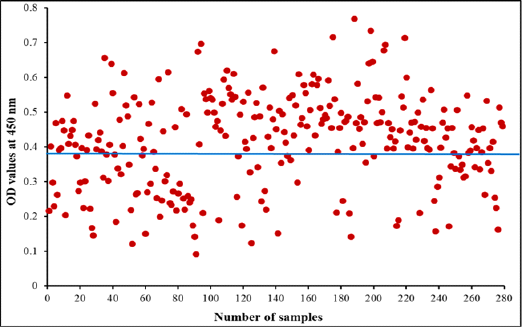
Figure 7: Diagnostic potency of purified fraction for camel Cephalopina nasal myiasis by indirect ELISA. The blue line represents cut off at 0.390 in collected random samples
Diagnostic Potency of larval fraction ELISA in camel Cephalopina nasal myiasis:
Indirect ELISA was performed to diagnose Cephalopina nasal myiasis in camels’ sera using purified fraction as antigen. The fraction detected 184 positive samples of 279 (65.95%) of examined camel random serum samples were infected with C. titillator and 95 were negative the OD values ranged from 0.092 to 0.769 as shown in Figure 7.
Discussion
Due to the importance of camels and the seriousness of disease that represents a serious veterinary problem worldwide and the difficulties to detect infestation with C. titillator in live animals, besides that it is difficult to differentiate between other nervous or respiratory pathogens, and infestation with C. titillator larvae which has the same clinical signs. And C. titillator larvae can’t be seen by blood or in fecal samples. There are no available commercial kits for the diagnosis of myiasis in camels. Immunodiagnosis to the detection of anti- C. titillator antibodies is an alternative method to clinical parasitological examination and postmortem examination.
Therefore, the present research concerning serological diagnosis of infestation in living animal.
Where, in the present research the results of the postmortem examination of slaughtered camels’ heads revealed 32 positives. The number of recovered C. titillator larvae in each head ranged from 17 to21 with a mean number 19 ± 2 larvae ⁄ animal, most larvae were attached to the mucosa of the nasopharyngeal cavity. Whereas, AL-Ani and Amr (2016) reported the number of recovered larvae ranged from 12 to 113, and average was 43 larvae in the nasopharynx, and number of recovered larvae reflects infection intensity. Furthermore, Yousef et al. (2016) reported that the intensity of infestation and the lesions induced by second and third larvae of C. titillator stimulate the immune response in camels. So that the immunodiagnosis in sera has been accurate and necessary for detected the infestation in living animals.
Therefore, the target of this research will be achieved by isolation of antigen with high specificity and sensitivity for accurate diagnosis of nasal myiasis. Additionally, in the present research, the gold standard control positive sera (collected from camels their nasopharyngeal cavities infested with C. titillator larvae), and gold standard control negative sera (collected from young camels less than three months and C. titillator-free) were used in for evaluating the diagnostic potency of the antigen used for screening infestation of larvae C. titillator in camels.
In the present research, specific larval fraction was isolated from the most potent diagnostic antigen; crude extract 2nd larval instar of C. titillator, as proved by ELISA. The purified fraction with most antigenic activities 87.67% of 2nd larval instar crude was isolated by immunoaffinity column chromatography with 1719.6 purification folds than crude extract. The success of the purification process was further supported by the low antigenic activity in the unbound fraction which means that the most antigenic activity was associated with the pure bound fraction. electrophoretic separation showed the purified fraction consists of two bands of molecular weight 52kDa and 22kDa. These two bands similar to bands 52 kDa and 22kDa were observed in SGc, while, one band at 51kDa was observed in DTc and ESP isolated antigens from second larvae instar of C. titillator (Yousef et al., 2015).
In the present study the fraction reacted strongly with positive naturally infected camel’s sera with C. titillator as proved by immunoblot analysis. Moreover, by indirect ELISA the fraction proved success in diagnosis recording 100% sensitivity and 96.88% specificity in the diagnosis of C. titillator infection in camels recording 65.95% infection. Whereas, previous study proved that the immunogenic bands, 58kDa and 35 KDa which extracted from the anterior end of second and third-stage larvae were used for diagnosis of Cephalopina infestation in the sera of living camels where band of 58 kDa recorded 100% sensitivity and band of 38kDa recorded 28.6% sensitivity (AL-Nasr et al., 2013). The specificity of both bands 58 and 28 didn’t extensively study so, the current research characterized by introducing a specific antigen with complete study of its sensitivity and specificity. Additionally, Attia et al. (2020), reported 100% sensitivity, 50% specificity, and 90% accuracy with crude salivary gland antigen of third larvae of C. titillator. The current fraction is the best in specificity compared with the other mentioned antigens.
ELISA proved potency in serodiagnosis of myiasis in the current research recording infection percentage of 65.95%. Previously, Yousef et al. (2016) measured antibody titers against both second and third antigens of C. titillator in camel mucus by ELISA and reported that the antibodies titers were higher in winter than in summer, and second larval instar, is the source of antigenic fractions (SGc, ESP, and DTc). Moreover, Hendawy et al. (2013) recorded prevalence percentages 51.72% and 53.57% with second larvae of C. titillator and SGc antigens, respectively by indirect ELISA. While, Attia et al. (2020) used indirect-ELISA for the detection and monitoring of C. titillator in camels, they reported 100% sensitivity, 50% specificity, and 90% accuracy with SGc antigen isolated from of third larval instar of C. titillator.
Collectively, the diagnostic purified fraction of 2nd larval instar of C. titillator could successfully be utilized for diagnosis of nasal myiasis by ELISA in camels which may help to control the infection and minimized transmission to human. Finally, a fraction was easily isolated with low costs and proved high diagnostic potency of camel nasal myiasis. And can be used for further studies of protection
acknowledgements
All authors extend their thanks and greatly appreciated to National Research Centre, Egypt for its support.
Conflict of interest
The authors declare that there is no conflict of interest in this research.
Authors Contribution
The authors participated in planning and study design. Nagwa I Toaleb collected Cephalopina titillator larvae from the nasal cavity, nasopharyngeal area, frontal sinuses, and turbinate bones of slaughtered camels’ heads and blood samples. Nagwa I Toaleb prepared crude antigens, performed Immunoaffinity chromatography of C. titillator 2nd instar larvae, SDS-PAGE, and western blot assays. Nagwa I Toaleb and Eman H. Abdel-Rahman shared in ELISA. Nagwa I Toaleb and Eman H. Abdel-Rahman shared in analyzed data and writing the manuscript.
References