Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (3): 171 – 176Assessment on Mithun, Jhum and their Interrelationship in Tribal Inhabitant Area of Papum Pare District, Arunachal Pradesh, India
Vasudevan Gowthaman1, Shambhu Dayal Singh1*, Kuldeep Dhama1, Rajamani Barathidasan1, Palani Srinivasan2, Nand Kishor Mahajan3, Muthannan Andavar Ramakrishnan3
- Avian Diseases Section, Division of Pathology, Indian Veterinary Research Institute, Izatnagar, Bareilly–243122, U.P
- Poultry Disease Diagnosis and Surveillance Laboratory, Veterinary College and Research Institute Campus, Namakkal– 637002
- Department of Veterinary Public Health, College of Veterinary Science, Hisar– 125004
- Division of Virology, Indian Veterinary Research Institute, Muktheswar, Uttarakhand – 263 138, India
*Corresponding author: sdsingh2005@rediffmail.com
ARTICLE CITATION:
Gowthaman V, Singh SD, Dhama1 K, Barathidasan R, Srinivasan P, Mahajan NK, Ramakrishnan MA (2014). Molecular characterization of chicken infectious anemia virus isolated from commercial poultry with respiratory disease complex in India. Adv. Anim. Vet. Sci. 2 (3): 171 – 176.
Received: 2014–01–19, Revised: 2014–02–20, Accepted: 2014–02–22
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.3.171.176
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
Chicken infectious anemia (CIA) is a highly contagious disease of young chicken, characterized by severe anemia, generalized lymphoid atrophy and increased mortality. The causative agent of the disease is Chicken Anaemia Virus (CAV), belonging to the genus Gyrovirus of the family Circoviridae. CAV has been included in the list of emerging and important viruses that are a severe threat to the Indian poultry industry. Although a few sequences of CAV from India are available in GenBank, no systematic analysis of Indian CAV strains has been performed to the best of our knowledge. Thirty–two commercial poultry flocks with a history of respiratory disease complex (RDC) from four different states of India were included in this study. Necropsy was carried out on freshly dead and ailing birds. Tissue samples were collected aseptically for direct tissue PCR detection of CAV. The PCR products were further subjected to sequencing to study the molecular epidemiology. PCR amplification of VP2 gene from the clinical tissues yielded expected product size of 419 bp in 30 out of 32 clinical cases screened. The Indian CAV viruses grouped with the major branch that consists of viruses from China, Brazil, USA, Malaysia, Bangladesh and Australia. The 16 Indian isolates shared 98.8 – 100 % homology among them. The percent identity matrix calculated for Indian isolates with CAV isolates from various parts of the world indicated closest relationship with isolates from China, USA and Australia (98.5–99.7 %), whereas Brazil, Malaysia and Bangladesh shared (98.8–100%) homology with Indian isolates. Further epidemiological and molecular studies are suggested to know the magnitude of infection and design appropriate disease prevention and control strategies for this economically important pathogen of poultry.
INTRODUCTION
Chicken infectious anemia (CIA) is a highly contagious disease of young chicken, characterized by severe anemia, generalized lymphoid atrophy, stunted growth and increased mortality (Todd, 2004; Dhama et al., 2008). The causative agent of the disease is Chicken Anaemia Virus (CAV), belonging to the genus Gyrovirus of the family Circoviridae. The CAV is one of the smallest, non-enveloped virus; it is 23–25 nm in size, icosahedral, having a 2.3 Kbp circular single stranded DNA genome. The genome codes for three viral proteins (VP1, VP2 and VP3) from single major transcript of 2.0 kbp size from three overlapping reading frames (ORF1, 2 and 3). VP1 and VP2 are the targets of neutralizing antibodies. In general, no significant antigenic or pathogenic difference was reported among the CAV isolates, only one serotype was recognized previously. However, an antigenically different isolate (CAV–7) has been reported from USA (Spackman et al., 2002). This virus was first reported by Yuasa et al. (1979) from contaminated vaccines in Japan. The chicken is the only natural host for the virus, which is ubiquitous not only in commercial poultry but also in SPF stocks (Cardona et al., 2000). Though chickens of all ages are susceptible to CAV infection, the clinical disease is mainly noticed in young chicks of upto 3–4 weeks of age, which usually acquire the infection vertically (Pope, 1991; Todd, 2000; Dhama et al., 2008) but after 3 weeks of age susceptibility to clinical disease decreases. CAV–infected birds develop a profound immunosuppression in the presence of concurrent infection with other viruses such as Marek’s disease virus (MDV) (Miles et al., 1999), Fowl adenovirus (FAV) (Toro et al., 2001), reoviruses (McNeilly et al., 1995) and NDV (De Boer et al., 1994), leading to synergistic effects of both agents (Pope, 1991); and also causes decreased immune response against several vaccine viruses, resulting in vaccination failures or aggravation of the residual pathogenicity of attenuated vaccine viruses (Todd, 2000; Schat, 2003; Toro et al., 2006; Dhama et al., 2008).
In India, the disease has long been suspected, on the basis of clinical manifestations and lesions (Verma et al., 1981; Khanna, 2010), virus detection by immunoperoxidase test, PCR and isolation. The disease has been reported from poultry flocks of some states of the country, and included in the list of emerging and important viruses that are a severe threat to the Indian poultry industry (Venugopalan et al., 1994; Kataria et al., 1999; Verma et al., 2005; Natesan et al., 2006; Praveen et al., 2008; Bhatt et al., 2011; Wani et al., 2013). Although a few sequences of CAV from India are available in GenBank (NCBI), no systematic analysis of Indian CAV strains has been performed to the best of our knowledge. Therefore, need was felt for further molecular characterization of these viruses to find out the genetic variation among them, if any, which would help devising suitable control strategy to prevent losses by this virus in the poultry industry. Sequencing was used as a tool to study the molecular epidemiology of Indian CAV isolates with the history of respiratory disease complex (RDC) in the present study.
MATERIALS AND METHODS
Field Samples
Thirty–two commercial poultry flocks with a history of respiratory disease complex (RDC) during May 2010 to September 2011 from four different states of India viz. Uttar Pradesh, Haryana, Rajasthan, and Tamil Nadu were included in this study. Selected flocks consisted of multi–aged layers and broilers and they were reared under cage system and deep litter system, respectively. The age of the RDC affected poultry birds of the 32 poultry flocks under investigation ranged between 4 and >72 weeks. All the birds were reared under standard managemental conditions recommended by the breeding companies. All the poultry flocks were vaccinated against respiratory pathogens viz, Newcastle disease, infectious bronchitis, infectious coryza and fowl cholera.
Collection and Transportations of Clinical Samples
Necropsy was carried out on freshly dead and ailing birds with symptoms of RDC. Tissue samples such as trachea, lungs, and spleen were collected aseptically for direct tissue PCR detection of CAV. The tissue samples were transported on ice from the field to Avian Diseases Section of the Division of Pathology, IVRI, Izatnagar. The samples were properly labelled and stored at –20 °C until used for PCR testing.
Polymerase Chain Reaction and Sequencing
The clinical tissues were cut in to small pieces and 5% homogenates were prepared in phosphate buffered saline (pH 7.2). Viral DNA from tissue homogenate was extracted using Wizard® Genomic DNA Purification Kit (Promega, USA) as per the manufacturer’s instructions. The primer pair targeting the VP2 gene of CAV viz., CAV_P1– 3'CTA AGA TCT GCA ACT GCG GA5' and CAV_P2 3'CCT TGG AAG CGG ATA GTC AT5' were used in the PCR detection to amplify a CAV virus specific 419–bp fragment (Ottiger, 2010). The amplification was carried out using PCR Master Mix (2X) (Fermentas, USA), with an initial denaturation at 95º C for 3 minutes, followed by 35 cycles at 94 °C for 1 min, 60 °C for 1 min, 72 ºC for 1 min, and a final extension at 72 °C for 10 min. Ten micro liters of the PCR products were analyzed by electrophoresis in 1.5% agarose gel with 0.5 mg/mL ethidium bromide. PCR products were purified by using ExoSAP–IT® (Affymetrix, USA) and sequenced using BigDye Terminator v3.1 kit (Applied Biosystem, USA) as per the manufacturer’s instructions.
Phylogenetic Analysis
The nucleotide sequence data generated were edited and aligned using Sequencing Analysis Software v5.3 (Applied Biosystems, USA) and MEGA 5 Softwares. Reference sequences were downloaded from NCBI database. Phylogenetic analysis and evolutionary associations were inferred in MEGA 5.0 using the Maximum Likelihood algorithm with Kimura–2P correction and 1000 bootstrap replications.

Figure 4: CAV–specific PCR amplicons visualized by agarose gel electrophoresis. Lane M, molecular weight marker; lanes 1 to 16, clinical samples; Lane 17– Negative control, and 18– positive control; Lanes 1–16 show the CAV specific 419 base pairs (bp) product considered as positive.
PCR Detection and Phylogenetic Analysis
PCR amplification of VP2 gene from the pooled tissues with primer pair described in materials and methods yielded expected product size of 419 bp (Figure 4). CAV specific nucleic acid was detected in 30 out of 32 clinical cases screened. Phylogenetic tree that was constructed using MEGA 5 program with 29 viruses available in the Gen Bank indicated multiple groups. The Indian CAVs were grouped with the major branch that consists of viruses from China, Brazil, USA, Malaysia, Bangladesh and Australia (Figure 5). The 16 Indian isolates shared 98.8 – 100 % homology among them. The percent identity matrix calculated for Indian isolates with CAV isolates from various parts of the world indicated closest relationship with isolates from China, USA and Australia (98.5–99.7%), where as Brazil, Malaysia and Bangladesh shared (98.8–100%) homology with Indian isolates.
RESULTS
Clinical Signs
Affected birds showed dullness, depression, somnolascence, stunted growth, prostration, facial swelling, decreased feed intake and water consumption, hock sitting posture, staggering gait, watery diarrhea, cyanotic combs and wattles, and drop in egg production. The respiratory signs including of sneezing, gasping, coryza and rales were indicative of involvement of the respiratory system. One of the pre–layer flock aged 17 weeks old out of the 32 flocks investigated showed signs of pale bird syndrome along with respiratory distress.
Gross Lesions
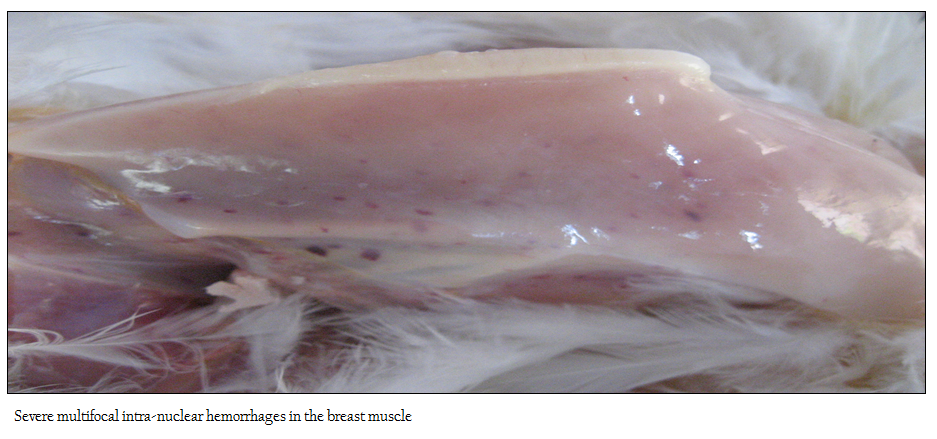
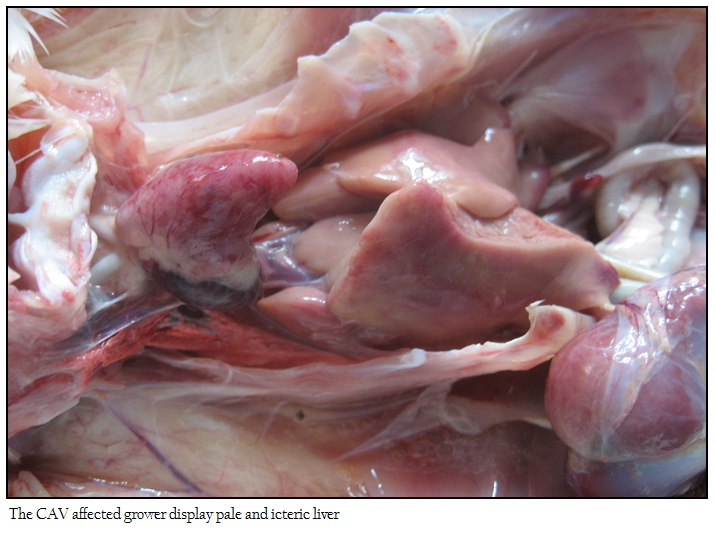
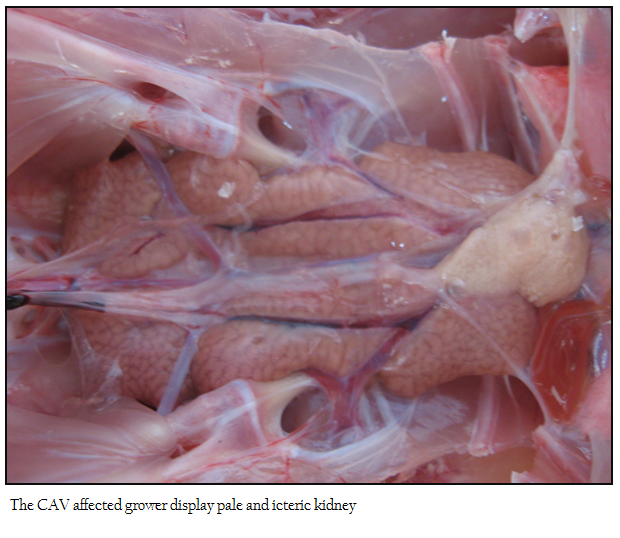
No appreciable/ minimal lesion was found in majority of the birds that died in peracute disease. Many affected birds showed emaciation, haemorrhagic/catarrhal tracheitis, diffuse pulmonary congestion and oedema, airsacculitis, fibrinous adhesive pericarditis and fibrinous perihepatitis, petechiae on the epicardial surfaces and abdominal fat, haemorrhagic proventriculitis, egg peritonitis, oophoritis, spleenic atrophy and or mottling and nephritis–nephrosis complex. The flock affected with pale bird syndrome displayed highly pale and icteric carcass with severe multifocal intramuscular haemorrhages in the pectoral region (Figure 1), and thigh muscles, widespread petechial haemorrhages on the junction of proventriculus and gizzard with increased mucous content. The liver, kidney and bone marrow were markedly pale and icteric (Figure 2 and 3)
Other Respiratory Agents
The samples were also positive for several respiratory agents like, FAV, LPAI, NDV, ILT, MG and MS, the data for which is not shown.
DISCUSSION
CAV has been reported from major poultry producing countries of the world including India and is being documented as emerging and an economically important pathogen from several countries (Kataria et al., 2005; Dhama et al., 2008; Schat, 2009; Oluwayelu, 2010; Bhatt et al., 2011; Snoeck et al., 2012; Gowthaman et al., 2013; Nayabian and Mardani, 2013; Wani et al., 2013). Recently, Bhatt et al. (2011) reported high prevalence (86.88%) of CAV antibodies in Northern India while Wani et al. (2013) reported a prevalence rate of 73.3% by PCR detection of CAV from clinical samples from different states of the country. The present study reports the molecular characterization of CAV isolates obtained from few states of India. Out of the 32 clinical samples screened by PCR, 30 were positive for CAV. Overt clinical signs of CAV could be recorded in a single farm only. All the cases were associated with RDC, this denotes that CAV is ubiquitous and mainly cause subclinical infection in India. Because of the widespread practice of vaccination for breeders and the presence of maternal antibodies, the clinical form of CAV is rare today (Sommer and Cardona, 2003). Although CAV is present as subclinical infections, in concurrence with other respiratory pathogens as identified in the present study, it measurably decreased the flock performance, increased the incidence of vaccine failures and other immunosuppressive and respiratory pathogens. Similar observations were also reported by several researchers in subclinical cases of CAV with other agents (De Boer et al., 1994; Toro et al., 2000). Close phylogenic relationship of Indian CAV isolates with USA, Brazil and China which are the leading poultry producers of the world denotes that the source of virus might have evolved from a common origin and circulating in East Asia and Australia; it could be either infecting grant parent stock or contaminated vaccines (Brown et al., 2000). The probable mechanism of transmission in Indian conditions could be vertical transmission from breeders or vaccine contamination, and CAV has been reported in SPF flocks and breeders in many occasions (Engström, 1999). CAV is an important extraneous pathogen that potentially contaminates the avian virus vaccines, particularly those prepared by inoculation of chicken embryos; CAV was detected as contaminant of NDV and IBD vaccines which were produced from embryonated chicken eggs (ECEs) (Aamir et al., 2007). Further extensive epidemiological studies are suggested for the virus in the country to know the magnitude of this important poultry virus, role of subclinical CAV infection in RDC along with isolation of viral strains and pathological studies. These would clearly define the disease status and need for the inclusion of CAV virus vaccination programs in India, and strengthening of R&D activities for development of rapid, sensitive and confirmatory diagnostics and modern generations vaccines to counter this economically important disease of poultry.
REFERENCES
Aamir UB, Wernery U, Ilyushina N, Webster RG (2007). Characterization of avian H9N2 influenza viruses from United Arab Emirates 2000 to 2003. Virol. 361: 45 – 55.
http://dx.doi.org/10.1016/j.virol.2006.10.037
PMid:17157891 PMCid:PMC2735206
Bhatt P, Shukla SK, Mahendran M, Dhama K, Chawak MM, Kataria JM (2011). Prevalence of chicken infectious anaemia virus (CIAV) in commercial poultry flocks of northern India: A serological survey. Transbound. Emerg. Dis. 58: 458 – 460.
http://dx.doi.org/10.1111/j.1865-1682.2011.01215.x
PMid:21414182
Brown HK, Browning GF, Scott PC, Crabb BS (2000). Full–length infectious clone of a pathogenic Australian isolate of chicken anaemia virus. Aust. Vet. J. 78: 637 – 640.
http://dx.doi.org/10.1111/j.1751-0813.2000.tb11942.x
PMid:11022294
Cardona CJ, Oswald WB, Schat KA (2000). Distribution of chicken anaemia virus in the reproductive tissues of specific–pathogen–free chickens. J. Gen. Virol. 81: 2067 – 2075.
PMid:10900046
De Boer GF, Van Roozelaar DJ, Moormann RJ, Jeurissen SH, Wijngaard JC, Hilbink F, Koch G (1994). Interaction between chicken anaemia virus and live Newcastle disease vaccine. Avian Pathol. 23: 263 –275.
http://dx.doi.org/10.1080/03079459408418994
PMid:18671091
Dhama K, Mahendran M, Somvanshi R, Chawak MM (2008) Chicken infectious anemia virus: an immunosuppressive pathogen of poultry – A review. Indian J. Vet. Pathol. 32: 158 – 167.
Engström BE (1999). Prevalence of antibody to chicken anaemia virus (CAV) in Swedish chicken breeding flocks correlated to outbreaks of blue wing disease (BWD) in their progeny. Acta Vet. Scand. 40: 97 – 107.
PMid:10605126
Gowthaman V, Singh SD, Dhama K, Barathidasan R, Kumar AM, Desingu PA, Mahajan NK, Ramakrishnan MA (2013) Fowl Adenovirus (FAdV) in India: evidence for emerging role as primary respiratory pathogen in chickens. Pak. J. Biol. Sci., 15: 900 – 903
http://dx.doi.org/10.3923/pjbs.2012.900.903
Kataria JM, Suresh RP, Verma KC, Toroghi R, Kumar NS, Kataria RS, Sah RL (1999). Chicken infectious anemia (CIA) in India: detection of the agent by polymerase chain reaction and transmission study. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 20: 91 – 95.
Kataria JM, Mohan MC, Dey S, Dash BB, Dhama K (2005). Diagnosis and immunoprophylaxis of economically important poultry diseases: a review. Indian J. Anim. Sci. 75(5): 555 – 567.
Khanna SK (2010). Emerging diseases of poultry: newer and cheaper methods of diagnosis and treatment. Poult. Technol. 5: 68 – 71.
McNeilly F, Smyth JA, Adair BM, McNulty MS (1995). Synergism between chicken anemia virus (CAV) and avian reovirus following dual infection of 1–day–old chicks by a natural route. Avian Dis. 39: 532 –537.
http://dx.doi.org/10.2307/1591806
PMid:8561738
Miles AM, Anderson AS, Bernberg EL, Kent J, Rosenberger JK, Pope CR, Morgan RW (1999). Comparison of two serotype 1 MDV isolates. Acta Virol. 43: 102 – 105.
PMid:10696428
Natesan S, Kataria JM, Dhama K, Rahul S, Baradhwaj N (2006). Biological and molecular characterization of chicken anemia virus isolates of Indian origin. Virus Res. 118: 78 – 86.
http://dx.doi.org/10.1016/j.virusres.2005.11.017
PMid:16384622
Nayabian H, Mardani K (2013) Molecular characterisation of the chicken anemia viruses isolated from broiler farms of west Azerbaijan, Iran. Avian Pathol. 42(2): 108 – 113.
http://dx.doi.org/10.1080/03079457.2013.766668
PMid:23581437
Oluwayelu DO (2010) Diagnosis and epidemiology of chicken infectious anemia in Africa. African J. Biotechnol. 9: 2043 – 2049
Ottiger HP (2010). Development, standardization and assessment of PCR systems for purity testing of avian viral vaccines. Biologicals. 38: 381 –388.
http://dx.doi.org/10.1016/j.biologicals.2010.01.015
PMid:20338785
Pope CR (1991). Chicken anemia agent. Vet. Immunol. Immunopathol. 30: 51 – 65.
http://dx.doi.org/10.1016/0165-2427(91)90008-Z
Praveen BN, Dhama K, Kataria JM, Dash BB, Singh SD (2008). Detection and isolation of chicken anemia virus from field samples of poultry flocks from Gujarat and Andhra Pradesh, India. Indian J. Comp. Microbiol. Immunol. Infect. Dis. 29: 23 – 26.
Schat KA (2003). Chicken infectious anemia. In: Saif, Y.M., H.J. Barnes, J.R. Glisson, A.M. Fadly, L.R. Mcdougald, and D.E. Swayne (eds), Diseases of Poultry, 11th edn. pp: 182 – 202.
Schat KA (2009). Chicken infectious anaemia. Iowa State University Press, Ames, USA. Curr. Topics Microbiol. Immunol. 331: 151 – 183.
http://dx.doi.org/10.1007/978-3-540-70972-5_10
PMid:19230563
Snoeck CJ, Komoyo GF, Mbee BP, Nakouné E, Le Faou A, Okwen MP, Muller CP (2012) Epidemiology of chicken anemia virus in Central African Republic and Cameroon. Virol. J. 9: 189.
http://dx.doi.org/10.1186/1743-422X-9-189
PMid:22958546 PMCid:PMC3495741
Sommer F, Cardona C (2003). Chicken anemia virus in broilers: dynamics of the infection in two commercial broiler flocks. Avian Dis. 47: 1466 –1473.
http://dx.doi.org/10.1637/7048
PMid:14708998
Spackman E, Cloud SS, Pope CR, Rosenberger JK (2002). Comparison of a putative second serotype of chicken infectious anemia virus with a prototypical isolate I Pathogenesis. Avian Dis. 46: 945 – 955.
http://dx.doi.org/10.1637/0005-2086(2002)046[0956:COAPSS]2.0.CO;2
http://dx.doi.org/10.1637/0005-2086(2002)046[0945:COAPSS]2.0.CO;2
Todd D (2000). Circoviruses: immunosuppressive threats to avian species: a review. Avian Pathol. 29: 373 –394.
http://dx.doi.org/10.1080/030794500750047126
PMid:19184829
Todd D (2004). Avian circovirus diseases: lessons for the study of PMWS. Vet. Microbiol. 98: 169 –174.
http://dx.doi.org/10.1016/j.vetmic.2003.10.010
PMid:14741130
Toro H, Gonzalez C, Cerda L, Hess M, Reyes E, Geissea C (2000). Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/ hydropericardium syndrome. Avian Dis. 44: 51 – 58.
http://dx.doi.org/10.2307/1592507
PMid:10737644
Toro H, González O, Escobar C, Cerda L, Morales MA, Gonzalez C (2001). Vertical induction of the inclusion body hepatitis/ hydropericardium syndrome with fowl adenovirus and chicken anemia virus. Avian Dis. 45: 215 – 222.
http://dx.doi.org/10.2307/1593031
PMid:11336070
Toro HS, Ewald, Hoerr FJ (2006). Serological evidence of chicken infectious anemia virus in the United States at least since 1959. Avian Dis. 50: 124 – 126.
http://dx.doi.org/10.1637/7442-092205R.1
PMid:16617995
Venugopalan AT, Elankumaran S, Raj GD, Manohar BM, Thangavelu A (1994). Isolation of chicken anemia virus in Tamil Nadu. Indian Vet. J. 7: 411.
Verma KC, Panisup AS, Mohanty GC, Reddy BC (1981). Infectious bursal disease (Gumboro–disease) and associated condition in poultry flocks of Andhra Pradesh. Indian J. Poult. Sci. 16: 385 – 392.
Verma S, Katoch RC, Mahajan A, Sharma M, Katoch V, Kataria JM, Dhama K (2005). Confirmation of an outbreak of chicken infectious anemia in organized poultry farm by polymerase chain reaction. Indian Vet. J. 82: 119 – 122.
Wani MY, Dhama K, Barathidasan R, Gowthaman V, Tiwari R, Bhatt P, Mahajan NK, Chawak MM, Singh SD, Kataria JM (2013). Molecular detection and epidemiology of chicken infectious anaemia virus in India. South Asian J. Exp. Biology. 3(4): 145 ‐ 151.
Yuasa N, Taniguchi T, Yoshida I (1979). Isolation and some characteristics of an agent inducing anemia in chicks. Avian Dis. 23: 366 – 385.
http://dx.doi.org/10.2307/1589567