Advances in Animal and Veterinary Sciences
Research Article
Sodium Butyrate and Rosemary Leaf Meal Inclusion in Broiler Diet: Effects on Gut Micro-Floral, Growth Performance, Ileum, Jejunum and Duodenal Histological Traits
Mercy C. Ogwuegbu1*, Augustine O. Ani1, Chika E. Oyeagu2, Charles O. Osita1, Uchenna Oyeagu3,4, Wilfred I. Ugwuoke5, Francis B. Lewu2
1Department of Animal Science, University of Nigeria Nsukka, Nigeria; 2Department of Agriculture, Faculty of Applied Sciences, Cape Peninsula University of Technology, Wellington Campus, Private Bag X8, Wellington 7654, Cape Town, South Africa; 3Department of Microbiology, University of Nigeria Nsukka, Nigeria; 4South East Zonal Biotechnology Center, University of Nigeria Nsukka, Nigeria; 5Department of Veterinary Anatomy, Faculty of Veterinary Medicine, University of Nigeria, Nsukka 410001, Enugu state, Nigeria.
Abstract | The gut histological traits, gut micro-flora and growth parameters of boiler birds fed different inclusion levels of sodium butyrate and rosemary leaf meal was investigated. A total of 320 one-day “Arbor acre strain” broiler chicks were allotted to 10 dietary treatments with 4 replicates of 8 birds each. The treatments includes : T1 = Basal diet (BD: Negative control); T2 = BD + 1g/Kg diet of Oxytetracycline (positive control); T3 = BD+2g sodium butyrate (SB) /kg diet; T4 = BD+4g SB/Kg diet; T5 = BD + 2.5g rosemary leaf meal (RLM) /kg diet; T6 = BD +5.0g RLM/kg diet; T7 = BD +2g SB + 2.5g RLM/kg diet; T8 = BD + 2g SB +5.0g RLM/kg diet; T9 = BD + 4g SB + 2.5g RLM/kg diet; and T10 = BD + 4g SB + 5.0g RLM/kg diet. The results showed that birds fed T3, T4, and T5 had the highest (p<0.05) body weight with an improved feed conversion ratio in both trial phases (starter and finisher). During the starter phase, the highest (p<0.05) villus length, crypth depth, thickness of the epithelium, thickness of muscularis in duodenum, jejunum and ileum was recorded for birds fed T4. During the finisher phase, the villus length, crypt depth, thickness of epithelium and muscularis of the duodenum, jejunum and ileum were higher (p<0.05) for birds fed 4g/kg sodium butyrate and 5g/kg rosemary leaf meal (except for crypth depth of jejunum and ileum). At the end of the starter phase, the data for the Lactobacillus counts of the ileum was highest (p<0.05) for birds fed dietary 4g/kg sodium butyrate and 5g/kg rosemary leaf meal, while, the Lactobacillus counts of the caecum was highest (p<0.05) for birds fed 4g/kg sodium butyrate, 2.5g/kg rosemary leaf meal and 5g/kg rosemary leaf meal. The highest (p<0.05) E.coli and Salmonella populations in both ileum and caecum during the starter phase was seen in birds fed the negative control diet. At the end of the finisher phase, the highest (p<0.05) proliferation of Lactobacillus in the ileum section of the gut was recorded for birds fed 2g/kg diet sodium butyrate, 4g/kg sodium butyrate, 2.5g/kg rosemary leaf meal, 5g/kg rosemary leaf meal and 4g/kg sodium butyrate +2.5g/kg rosemary leaf meal, while Lactobacillus counts in the caecum was highest (p<0.05) for birds fed 2g/kg diet sodium butyrate, 4g/kg sodium butyrate, 2.5g/kg rosemary leaf meal and 5g/kg rosemary leaf meal. The populations of Lactobacillus were lowest (p<0.05) in both ileum and caecum parts of the gut for birds fed negative control (T1) diet, while the E.coli and Salmonella counts in the caecum were highest (p<0.05) for birds fed the negative control (T1) diet compared with other dietary treatments. It was concluded that T4 and T6 had a better gut integrity and improved histological traits. Moreover, 2g/kg diet sodium butyrate, 4g/kg sodium butyrate or 2.5g/kg rosemary leaf meal can be used safely for a better performance as they enhanced the growth traits of the birds.
Keywords | Broilers, Gut histology, Micro-flora, Organic acids, Rosemary.
Received | April 02, 2021; Accepted | April 17, 2021; Published | June 15, 2021
*Correspondence | Mercy C Ogwuegbu, Department of Animal Science, University of Nigeria Nsukka, Nigeria; Email: mercy.ogwuegbu@unn.edu.ng
Citation | Ogwuegbu MC, Ani AO, Oyeagu CE, Osita CO, Oyeagu U, Ugwuoke WI, Lewu FB (2021). Sodium butyrate and rosemary leaf meal inclusion in broiler diet: effects on gut micro-floral, growth performance, ileum, jejunum and duodenal histological traits. Adv. Anim. Vet. Sci. 9(7): 1095-1112.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1095.1112
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ogwuegbu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Nutrition, health, and management concerns form key factors determining animal growth and productivity in the global poultry industry, and in particularly the developing countries. Health and productivity in broiler birds can be greatly enhanced through their dietary supplementations with appropriate nutrients and additives. According to Yue et al. (2018), nutrient digestion, absorption and utilization for improved diseases resistance are largely dependent on the gut health and morphology of birds. In addition to its use in the treatment of various diseases, antibiotics have been long used as a growth stimulating agent given its role in the modulation of the gut ecosystem (Mashael et al., 2020). However, the use of this antibiotics in animal production was banned by the European Union in 2006, following increasing concerns of antibiotic resistance issues (in animals and man who consume its products), in addition to its other negative health effects such as food poisoning, muscle tremors, and tachycardia (Hassan et al., 2018; Ma et al., 2019). Consequently, efforts have been directed towards the identification and use of healthy natural alternatives to antibiotics in animal production (Lum et al., 2018). In line with this, several studies have been investigated and reported, and these include organic acids (Sohail and Javid 2016; Zulfqarul et al 2017; Ogwuegbu et al., 2021), herbal products (Nikola et al., 2016; Lipiński et al., 2019), natural spices and polyphenols (Hayajneh 2019; Nikola et al., 2020; Ogwuegbu et al., 2021), essential oils (Yang et al., 2019; Pham et al., 2020), probiotics (Al-khalaifa et al., 2019), prebiotics (Rehman et al., 2020) and enzymes (Oyeagu et al., 2016; Oyeagu et al., 2019).
Organic acids are weak acid compounds with pH values ranging from three (carboxylic) to nine (phenolic), which are widely distributed in nature and found in animals, plants and microbial substances (Papagianni, 2011). Among these acids, short chain fatty acids (SCFAs) including acetic acid, propionic acid and butyric acids, are volatile fatty acids (VFAs) produced through gut microbial fermentation, which have been noted for its nutritional importance. These acids are known to selectively stimulate the proliferation of beneficial micro organism, while inhibiting the growth of pathogenic ones within the gut system of the birds (Ahsan et al., 2016). Butyric acids with a molecular weight of 88.12g/mol, density 0.958g/ml, and pH 4.82 is particularly noted for its unpleasant odour, excessive volatility and corrosive nature (Arbab et al., 2017). However, the sodium salts of butyric acid are commonly used in animal feeding due to their stability, easy of use, and relatively less odour (Ahsan et al., 2016).
Dietary inclusion of sodium butyrate have been reported to show some benefits on birds intestinal villus surface, intra-luminal digestibility of mineral and proteins, weight gain, carcass traits, and immune performances of broilers (Qaisrani et al., 2015; Sikandar et al., 2017; Abonyi et al., 2020). Sodium butyrate is easily transformed into butyric acid in the intestine, where it enhances the intestinal health through various mechanisms such as decreasing intestinal susceptibility to pathogenic bacteria colonization (Soad et al., 2016), and increasing growth performance of the birds under stress (Wu et al., 2018). The inclusion of sodium butyrate in poultry diet has been linked to enhanced immune function (Zhou et al., 2017); development of gut wall tissues, as it promotes the growth of symbiotic intestinal micro-flora (Wafaa et al., 2016; Ahsan et al., 2016); decreases the colonization of harmful bacteria in the digestive tract of broilers, improved body weight, feed conversion ratio and beneficial bacteria populations (Wu et al., 2016). Raza et al. (2019) recorded a significant positive effect of butyrate supplementation on broilers in keeping optimal gut health and morphology, and stimulating increase in growth performance. It is also an energy source with bacteriostatic and immune enhancement properties. Ahsan et al. (2016) and Moquet et al. (2016) also found that butyrate presence in the digesta of distinct gastrointestinal tract segments of broilers leads to differential effects on digesta retention time, gut morphology and preteolytic enzymatic activities, ultimately resulting in differences in protein digestibility.
On the other hand, phytogenic feed components are substances of plant origin added to animal diet to improve production and health, and were reported to have several positive effects on broilers (Raza et al., 2016; Abudabos et al., 2017). Rosemary leaf meal (Rosemarinus officinalis) is an aromatic herb that is used in the popularity prescription. It contains rosmanol, carnosol and their acid forms, or flavonoids as its natural major active compounds (Andrade et al., 2018). Rosemary is known to stimulate feed intake by the secretion of endogenous enzymes resulting in enhanced feed intake, as well as nutrient digestion and absorption from the gut (Tehseen et al. 2016; Ahsan et al., 2018). The beneficial features of rosemary leaf meal is predominantly derived from their bioactive molecules including carvacrol, thymol, capsaicin, cineole, etc. which are responsible for its antioxidant, antimicrobial, and antifungal properties (Ahsan et al., 2018). These herbal feed additives of plant origin are generally believed to be healthier, less dangerous and safer, with no dangerous residue found in meat. These properties are what make this photogenic feed addictives a suitable alternative to Antibiotic growth promoters.
Numerous research have independently investigated the dietary roles of butyric acid (Ahsan et al., 2016; Arbab et al., 2017; Deepa et al., 2017; Elnesr et al., 2019; Abonyi et
Table 1: Ingredient (%) and Chemical composition (g/kg DM) of experimental diets for broiler chicks at starter phase (0-4 weeks)
Ingredients (%)
|
T1 |
T2 |
T3 |
T4 |
T5 | Diets T6 |
T7 |
T8 |
T9 |
T10 |
| Maize | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 | 44.00 |
| Wheat offal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 |
| Groundnut cake | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 |
| Palm kernel cake | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish meal | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Bone meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vit-min premix* | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Methionine | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Lysine | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Butyrate | 0.00 | 0.00 | 0.20 | 0.40 | 0.00 | 0.00 | 0.20 | 0.20 | 0.40 | 0.40 |
| Rosemary | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 0.50 |
| Oxytetracycline | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Calculated Composition | ||||||||||
| Crude protein (%) | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 | 22.67 |
| Energy(Mcal/kg ME) | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 |
Crude fibre(%) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Chemical Composition | ||||||||||
| Moisture | 10.00 | 11.60 | 9.60 | 10.20 | 8.40 | 8.60 | 9.60 | 8.40 | 8.40 | 7.20 |
| Crude protein (%) | 21.56 | 22.00 | 21.96 | 21.78 | 21.89 | 21.15 | 22.85 | 21.08 | 22.00 | 21.96 |
| Ether extract (%) | 3.00 | 3.00 | 4.00 | 3.00 | 3.00 | 5.00 | 1.00 | 4.00 | 3.00 | 5.00 |
| Crude fibre (%) | 5.03 | 5.05 | 5.00 | 4.98 | 5.01 | 5.05 | 5.00 | 5.07 | 5.03 | 5.02 |
| Ash (%) | 4.00 | 6.00 | 7.00 | 9.00 | 5.00 | 6.00 | 5.00 | 5.00 | 8.00 | 4.00 |
| NFE (%) | 51.01 | 48.35 | 47.44 | 41.04 | 49.7 | 53.20 | 49.55 | 53.45 | 49.57 | 51.82 |
| CHO (%) | 56.04 | 53.40 | 52.44 | 46.02 | 54.71 | 58.25 | 54.55 | 58.52 | 54.60 | 56.84 |
Table 2: Ingredient (%) and Chemical composition (g/kg DM) of experimental diets for broiler chicks at finisher phase (4-8 weeks)
| Ingredients (%) |
T1 |
T2 |
T3 |
T4 |
T5 | Diets T6 |
T7 |
T8 |
T9 |
T10 |
| Maize | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 | 54.04 |
| Wheat offal | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 | 5.14 |
| Soybean meal | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 | 12.42 |
| Groundnut cake | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 | 16.00 |
| Palm kernel cake | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 | 5.4 |
| Fish meal | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Bone meal | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Vit-min premix* | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Methionine | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Lysine | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Butyrate | 0.00 | 0.00 | 0.20 | 0.40 | 0.00 | 0.00 | 0.20 | 0.20 | 0.40 | 0.40 |
| Rosemary | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.50 | 0.25 | 0.50 | 0.25 | 0.50 |
| Oxytetracycline | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Calculated Composition | ||||||||||
| Crude protein (%) | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Energy(Mcal/kg ME) | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 |
| Crude fibre(%) | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Chemical Composition | ||||||||||
| Moisture | 8.40 | 9.20 | 9.00 | 8.20 | 10.40 | 9.20 | 8.80 | 8.80 | 10.40 | 7.80 |
| Crude protein | 18.12 | 18.07 | 18.01 | 18.05 | 18.00 | 18.10 | 18.14 | 18.10 | 18.09 | 18.06 |
| Ether extract | 4.00 | 4.05 | 4.12 | 3.65 | 4.00 | 5.00 | 4.86 | 5.00 | 6.00 | 5.08 |
| Crude Fibre | 4.98 | 5.02 | 4.97 | 5.00 | 5.01 | 5.05 | 5.00 | 4.99 | 5.04 | 5.01 |
| Ash | 6.00 | 7.00 | 6.00 | 5.00 | 5.00 | 4.00 | 6.00 | 4.00 | 7.00 | 6.00 |
| NFE | 58.50 | 56.66 | 57.90 | 60.10 | 57.59 | 58.65 | 57.20 | 59.11 | 53.47 | 58.05 |
| CHO | 63.48 | 61.68 | 62.87 | 65.10 | 62.60 | 63.70 | 62.20 | 64.10 | 58.51 | 63.06 |
al., 2020) and rosemary leaf meals (Abudabos et al., 2018; Andrade et al., 2018; Saleh et al., 2018; Mashael, 2020) in birds. But, there is a paucity of information on the combined nutritional effects of those substances on health and performance of broiler birds. Consequently, this study was designed to assess the effect of sodium butyrate and rosemary leaf meal at both single and combined levels as an alternative to antibiotics on gut micro-floral, growth performance, ileum, jejunum, duodenal and histological traits of broiler chickens.
MATERIALS AND METHODS
Ethical Consideration
The experiment was carried out by the provisions of the Ethical Committee on the use of animals and humans for biomedical research of the University of Nigeria, Nsukka, Nigeria.
Study Site
The study was carried out at the Poultry Unit of the Department of Animal Science Teaching and Research Farm, University of Nigeria, Nsukka, Nigeria. Nsukka lies within longitude 6o 45′E and 7oE and latitude 7o12.5 ′N and on the altitude 447m above sea level. The climate of the study area is typically tropical, with relative humidity ranging from 65 to 80% and mean daily temperature of 26.8oC (Ogwuegbu et al., 2020).
Characteristics of sodium butyrate, rosemary leaf meal, and Oxytetracycline
Butyrate: The tested sodium butyrate (Gusto Bp70) was purchased from Agro Barmagen Nig Ltd, Ibadan; A subsidiary of Bar-Margen group Israel). The active substance in the butyrate is 40% free sodium butyrate, 30% protected sodium butyrate.
Rosemary leaf meal: The rosemary leaf meal was purchased from the main market Onitsha, Anambra State, Nigeria and has the active ingredients of 24 flavonoids (mainly flavones), 5 phenolic acids, 24 diterpenoids (carnosic acid, carnosol and rosmanol derivatives), 1 triterpenoid (betulinic acid) and 3 lignans (medioresinol derivatives).
Oxytetracycline: The tested Oxytetracycline (C22H24N2O9) (Tetracin® Vetindia Pharmaceuticals limited India; ®African Representative, Global Organics limited No 81A, Lamido Road, Kano, Nigeria) is a soluble powder of oxytetracycline hydrochloride 5%W/W. Each gram contains: Oxytetracycline Hydrochloride BP 50mg. Oxytetracycline is a synthetic antibiotics that was used to generate a broader assessment of its similarities and differences with the effects of sodium butyrate and Rosemary leaf meal used in the study.
Experimental diets
The feeding strategy consisted of starting (0-28 days) and finishing (29-56 days) basal diets (BD) (Tables 1 and 2), which were formulated to meet the birds’ dietary nutritional requirements (NRC, 2018; Oyeagu et al., 2019). At each feeding phase (starting and finishing), there were ten dietary treatment groups that contain different levels of sodium butyrate (SB), and rosemary meal (RM) as follows: T1= Basal diet (BD: Negative control) , T2 = BD + 1g/Kg diet of Oxytetracycline (Positive control), T3= BD +2g SB/kg diet, T4= BD + 4g SB/Kg diet, T5 BD + 2.5g RLM/kg diet, T6= BD + 5.0g RLM/kg diet , T7= BD +2g SB + 2.5g RLM/kg diet, T8= BD + 2g SB +5.0g RLM/kg diet, T9= BD + 4g SB + 2.5g RLM/kg diet and T10= BD + 4g SB + 5.0g RLM/kg diet. The chemical (Proximate) compositions of the experimental diets were analyzed according to the Association of Official Agricultural Chemists (AOAC, 2006; Idowu, 2020) methods.
Experimental birds and managements
A total of 320 one-day-old “Arbor acre strain” broiler chicks were used in the present study. Thirty-two (32) birds were assigned randomly to one of the ten experimental diets (T1, T2, T3, T4, T5, T6, T7, T8, T9 and T10). Each experimental diet was replicated into four experimental pens measuring 2.6m width X 3m length with eight birds each. The birds were housed in cages with fresh wood shavings as litter. General flock prophylactic management and routine vaccination were administered as follows; Day 1- intra ocular (New castle disease vaccine), week 2- Gumboro disease vaccine, week 3- Lasota (New castle disease vaccine), week 4-Gumboro disease vaccine, week 5-fowl pox vaccine, week 6-8, Lasota vaccine was repeated because of its prevalence in the farm. A stress pack was administered to the birds via drinking water at 100 g/50 liters (according to manufacturer’s recommendation) to boost appetite and energy supply. Dietary treatments and clean water were provided ad libitum in an eight-week feeding trial. The room temperature was monitored with the use of thermometer, and the lighting was provided using a 200 watt bulb.
Growth Performance
Average daily feed intake (ADFI) per bird was measured from day 1 to day 56 of age by subtracting the weight of the feed left over from the feed offered and dividing the difference by the total number of birds in the pen. The initial live-weight of the birds was measured at the beginning of the experiment. Thereafter, average live-weight was measured weekly by weighing all the birds in each pen using a 10.1kg capacity precision weighing balance (models A and D Weighing GK -10K industrial balance made in China. The feed conversion ratio was calculated as feed intake divided by the body weight.
Histological Study
Three birds were randomly selected from each replicates group at 28 and 56 d for the collection of small intestine samples. Immediately after euthanasia, the intestines were removed. Jejunum, duodenum and ileum samples was fixed in a “ Bouins fluid” (Pallav et al., 2016) for a minimum of 48 h, changed after 48 hours into a 30% phosphate-buffered formalin after which 4.0-μm sections was prepared. The sections was stained with standard haematoxylin–eosin solution and observed for villus height (VH), villus width (VW), crypt depth (CD), height of the epithelium and thickness of the tunica muscularis at 100× magnification by light microscope using a calibrated ocular micrometer.
Iluem and Cecal micro-flora composition
Three birds were selected at random per replicate at the age of 28 and 56 days and were euthanized. The abdominal cavity was opened, and the entire gastro intestinal tract was removed aseptically. All digesta contents of ileum and caecum were collected immediately under aseptic conditions into sterile glass bags and put on ice before they were transported to the laboratory for enumeration of microbial populations. Ceacal and ileum digesta contents were emptied aseptically in a new sterile bag and were immediately diluted 10-fold (ie 10% wt/vol) with sterile ice-cold anoxic PBS (0.1 m; pH 7.0) and subsequently homogenized for 3 min in a stomacher (Bagmixer 100 Minimix, Interscience, Arpents, France). Each ceacal and ileum digesta homogenate was serially diluted from 10-1 to 10-7. Dilutions were subsequently plated on duplicate selective agar media for enumeration of target bacterial groups. In particular, E. Coli, Lactobacillus spp were enumerated using VRB agar (MERCK, 1.01406), Rogosa agar (MERCK, 1.10660), and Beerens agar respectively according to (Tuohy et al., 2002; Oyeagu et al., 2019). Plates were incubated at 39 °C for 48 to 120 hours anaerobically (Beerens, Rogosa agars) or 24 to 48 hours anaerobically at 37 °C (VRB agar). The bacterial colonies were enumerated, and the average number of live bacteria was calculated based on the weight of original ileum and caecum contents. All quantitative data were converted into logarithmic colony forming units (cfu/g), (Koc et al., 2010; Oyeagu et al., 2019).
Statistical design and analysis
Data collected during the study were subjected to analysis
Table 3: The effect of Sodium butyrate and Rosemary meal on feed intake, body weight gain and feed conversion ratio of broiler birds (n=32, N=320)
| Diets | |||||||||||
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | SEM | |
|
Daily performance |
|||||||||||
| Initail weight (g) | 43.75 | 43.00 | 43.50 | 43.50 | 43.70 | 43.50 | 43.50 |
44. 00 |
43.75 | 43.50 | 0.07 |
| Daily feed intake (g) |
92.52c |
106.49ab |
113.01a |
103.55b |
108.86ab |
114. 11a |
109. 10ab |
107. 75ab |
107.’ 04ab |
114.78a |
1.40 |
| Daily weight gain (g) |
39.20c |
46.12ab |
48.32a |
47.24a |
48.04a |
46. 48ab |
43.4 8b |
46. 63ab |
44. 77b |
47.40a |
1.63 |
| Starter phase | |||||||||||
| Feed intake (g) |
1299.90c |
1620.00a |
1620.10a |
1569.90b |
1678.50a |
1630. 00a |
1589. 00b |
1526. 10b |
1544. 80b |
1541.50b |
19.28 |
| Body weight gain (g) |
621.41c |
745.19b |
826.81ab |
809.94ab |
883.75a |
845. 41a |
814. 56ab |
775. 35b |
782. 25b |
756.53b |
15.09 |
|
FCR (g g-1) |
2.09ab |
2.17a |
1.96b |
1.94b |
1.90c |
1.93b |
1.95b |
1.97b |
1.97b |
2.04ab |
0.02 |
| Finisher phase | |||||||||||
| Feed intake (g) |
3881.10d |
4343.60c |
4708.80b |
4229.20c |
4417.70bc |
4760. 00b |
4520. 80c |
4508. 00c |
4449. 40bc |
4886.20a |
65.56 |
| Body weight gain (g) |
1573.70d |
1837.40ab |
1879.20a |
1835.30ab |
1806.90ab |
1757. 70b |
1620. 70c |
1836. 10ab |
1724. 90b |
1897.90a |
28.30 |
|
FCR (g g-1) |
2.47bc |
2.36c |
2.51bc |
2.30d |
2.44bc |
2.71ab |
2.79a |
2.45bc |
2.58b |
2.57b |
0.03 |
| Overall phase | |||||||||||
| Feed intake (g) |
5181.00c |
5963.60ab |
6328.90a |
5799.10b |
6096.20ab |
6390. 00ab |
6109. 80ab |
6034. 10ab |
5994. 20ab |
6427.70a |
78.60 |
| Body weight gain (g) |
2195.11c |
2582.59b |
2706.01a |
2645 24a |
2690.65a |
2603. 11a |
2435. 26bc |
2611. 45a |
2507. 15b |
2654.43a |
34.90 |
|
FCR (g g-1) |
2.36b |
2.31b |
2.34b |
2.19c |
2.26c |
2.45ab |
2.51a |
2.31b |
2.39ab |
2.42ab |
0.02 |
abc Rows means with different superscripts differ significantly. SEM: Standard error of the mean, T1= Basal diet (BD: Negative control) T2 = BD + 1g/Kg diet of Oxytetracycline (Positive control), T3= BD + 2g SB/kg diet, T4= BD + 4g SB/Kg diet, T5 = BD + 2.5g RM/kg diet, T6= BD + 5.0g RM/kg diet , T7= BD + 2g SB + 2.5g RM/kg diet, T8= BD + 2g SB +5.0g RM/kg diet, T9= 4g SB + 2.5g RM/kg diet and T10= BD + 4g SB + 5.0g RM/kg diet.
of variance (ANOVA) for completely randomized design (CRD) as described by (Steel and Torrie, 1980) using general linear model Procedure of (SAS, 2010). The statistical model used to test the effects of treatment on gut micro-floral, growth traits, ileum, jejunum and duodenal histological traits was;
Yij = µ +Ai + Ʃij
Where:
Yij : observed value of a dependent variable.
µ: overall mean
Ai: effect of different sodium butyrate and rosemary leaf meal
Ʃij: residual error.
The differences between means were tested for significance at p<0.05 using least significant difference (LSD) range test.
RESULTS AND DISCUSSION
General performance data of broiler birds
The growth performance of broiler birds fed different inclusion levels of sodium butyrate and rosemary leaf meal at different phases is presented in Table 3. All the growth parameters considered in this study were significantly (P<0.05) affected by the treatments at all phases. Birds fed T1 (Control), recorded the lowest (P<0.05) daily feed intake as well as daily weight gain. The highest (p<0.05) daily feed intake was seen in birds fed T3, T6, and T10, even though they are statistically similar to those that received T2, T5, T7, T8 and T9. The daily weight gain was highest (p<0.05) for birds fed T3, T4, T5, and T10. During the starter phase, feed intake values was highest (p<0.05) for birds fed T2, T3, T5, and T6, while, birds fed T1 recorded the lowest feed intake. The lowest (p<0.05) value for body weight gain was seen in birds fed T1, while, the highest (p<0.05) value was observed in birds fed T5 and T6, though, statistically similar with those that received dietary T3, T4, and T7. Feed conversion ratio was better (p<0.05) for birds fed T5, compared with birds that received other treatment diets. The last phase of the feeding trial (finisher phase) showed that, birds fed T1 consumed less (p<0.05) feed with a poor body weight gain, while, those fed T10 had the highest feed intake. The body weight gain was highest (p<0.05) for birds fed T3 and T10, though, statistically the same with those that received dietary T2, T4, T5, and T8. A better (p<0.05) FCR value was seen in birds fed T4 compared with those in other treatments. The overall phase presents a higher (p<0.05) body weight gain recorded for birds fed T3, T4, T5, T6, T8, and T10, while a better (p<0.05) feed conversion to meat ratio was seen in birds fed T4, and T5. Birds fed T1 had the lowest (p<0.05) feed intake as well as body weight gain.
Generally, the dietary inclusion of sodium butyrate (2g SB/kg feed = T3; 4g SB/kg feed = T4) and rosemary leaf meal (2.5g RM/kg feed = T5) at single levels improved the BWG and FCR of broilers compared with the negative (no additive) control and the positive (antibiotic) control diet as well as the combination of sodium butyrate and rosemary meal. Non-antibiotic feed additives are now used to improve the growth and feed utilization (Alaeldein et al., 2018). The results of the present study suggested that sodium butyrate (organic acid) and rosemary leaf meal could replace antibiotics in broiler chicken’s diet and be used both at therapeutic and sub-therapeutic levels in animal feed for the treatment of diseases, and as a growth promoter to enhance production. It is assumed that the improved performance observed in this study may be due to the creation of the acidic environment in the gut after consumption of the supplemented diets which in turns decreases the load of pathogens thereby exerting its anti-microbial effects (Alaeldein et al., 2018). Also, the suggested mechanism of action of the sodium butyrate and rosemary leaf meal may be due to the enhanced feed intake, improved nutrient digestion, increased secretion of digestive enzymes and greater absorption in the intestines (Abudabos et al., 2016, 2017). The addition of sodium butyrate and rosemary leaf meal in the feed of broilers, probably, improves health, nutrient absorption and promotes growth rates of broilers, as well as improved feed to meat conversion rate (Abdelrahim et al., 2018; Ogwuegbu et al., 2020). Ahsan et al. (2016); Abonyi et al. (2020) suggested that organic acids improved intraluminal digestibility of mineral, protein and energy by reducing microbial competition with the host for nutrients and endogenous nitrogen losses, by lowering the incidence of sub-clinical infections and secretion of immune mediators, by reducing the production of ammonia and other growth depressing microbial metabolites. Probably these could be the reasons that butyrate improved feed utilization leading to a better performance in the birds. The results of this study is in agreement with the findings of Sikandar et al. (2017) who reported that the inclusion of sodium butyrate (SB) at 4g/kg in the diet of broiler chicken performed better than antibiotics in improving BWG of birds with a superior feed efficiency. According to Wu et al. (2018), sodium butyrate improved the body weight of broilers, and they attributed it to the beneficial effect of sodium butyrate in promoting the intestinal epithelium cell development and modulating intestinal symbiotic growth. The improved feed conversion ratio for birds fed 2g SB/kg feed (T3), and 4g SB/kg feed (T4) may be due to the effect of sodium butyrate as it increases the absorption of nutrients as well as the exclusion of harmful microbial load (Raza et al., 2019). Contrary to the results of this study, Wu et al. (2016) reported that sodium butyrate addition did not influence the body weight gain, Feed intake or feed conversion ratio. These variable results may be attributed to the available contents of the sodium butyrate (SB) addition and the type of microbial environment to which the chicks were exposed. It is important to note that the available content of the tested organic acid used in this study is made up of mono and diglycerides with approximately 80 % by weight of butyrate. According to Soad et al. (2016), the improved performance of broiler chickens fed dietary sodium butyrate may be attributed to better feed utilization through improved villus height. The improved villus height enhanced the villus function which leads to a better growth performance of the birds (Shaaban et al., 2020). Arbab et al. (2017) opined that better performance may be due to the creation of an acidic environment in the gut after SB consumption, which in turn minimized the load of pathogens (Arbab et al., 2017). The in-feed SB may improve the intraluminal digestibility of minerals and proteins which may result in improved weight gain in SB offered groups as mentioned by Ahsan et al. (2016). The result of the present study agreed with the findings of Abudabos et al. (2016) who reported that the supplementation of organic acids improved the FCR in broilers chicken. The study showed that 2 g/kg feed (T3), and 4 g/kg feed (T4) of microencapsulated sodium butyrate reduced feed intake with a positive body weight gain and feed conversion ratio throughout the feeding trial. Ahsan et al. (2016) opined that, butyric acid and its glyceride forms could cause feed intake depression, unlike propionates and acetates. The microencapsulation of sodium butyrate allowed for the targeted release of this compound at the ileum level and it directly affected the intestinal morphology, potentially the micro-biota, and digestive processes in this section of the gut. As reported by Kaczmarek et al. (2016), unprotected or un-encapsulated butyric acid salts (butyrate) are also rapidly absorbed in the upper parts of the GIT, thus, the protection of the active ingredient is crucial for these
Table 4: Effect of Sodium butyrate and Rosemary powder on histological traits of samples of duodenum, jejunum and ileum in broiler starter birds (n=32, N=320)
| Diets | |||||||||||
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | SEM | |
| Starter | |||||||||||
| Duodenum | |||||||||||
| Villus length (nm) |
1218.1c |
1348.1b |
1426.5a |
1475.1a |
1356.9b |
1391.6ab |
1305.2b |
1311.2b |
1308. 6b |
1317. 2b |
11.90 |
| Crypth depth (nm) |
203.50f |
232.18e |
259.14b |
271.18a |
250.11c |
261.70ab |
240.19e |
238.61e |
239. 15de |
240. 19de |
2.77 |
|
Thickness of epithelium (nm) |
43.14g |
56.79f |
60.91c |
70.11a |
62.32d |
68.78ab |
65.15b |
67.84ab |
66. 71bc |
59. 11ef |
1.23 |
| Thickness of muscularis (nm) |
154.82c |
171.97b |
176.04b |
184.25a |
175.18b |
182.11a |
175.08b |
174.94b |
173. 91b |
174. 57b |
1.28 |
| Jejunum | |||||||||||
| Villus length (nm) |
1107.5d |
1203.5c |
1325.1ab |
1392.3a |
1354.1ab |
1336.2ab |
1304.2b |
1319.2ab |
1328. 6ab |
1325. 1ab |
14.34 |
| Crypth depth (nm) |
135.16c |
157.11b |
161.72b |
178.26a |
160.18b |
169.14a |
158.13b |
157.78b |
158. 52b |
160. 08b |
1.71 |
|
Thickness of epithelium (nm) |
44.63d |
47.51c |
48.01c |
56.61a |
49.18c |
53.72b |
48.11c |
47.61c |
47. 72c |
49. 55c |
0.56 |
| Thickness of muscularis (nm) |
121.70d |
128.71c |
135.61ab |
138.79a |
136.11ab |
136.81a |
134.76abc |
132.61abc |
130. 11bc |
130. 34bc |
0.93 |
| Ileum | |||||||||||
| Villus length (nm) |
974.23c |
1164.4ab |
1104.6b |
1256.7a |
1008.2bc |
1248.2a |
1136.1b |
1146.1b |
1105. 3b |
1005. 3b |
19.64 |
| Crypth depth (nm) |
138.14e |
149.11d |
156.18b |
165.04a |
157.23b |
160.16ab |
154.72b |
149.75cd |
157. 19b |
149. 98cd |
1.16 |
|
Thickness of epithelium (nm) |
43.27d |
45.93c |
46.01c |
56.73a |
47.11c |
53.24ab |
45.34cd |
46.71c |
47. 06c |
45. 82c |
0.65 |
| Thickness of muscularis (nm) |
167.85d |
172.11c |
171.56c |
178.76a |
172.31c |
176.54ab |
173.11c |
174.01bc |
172. 01c |
173. 05c |
0.53 |
a,b,c,d,e,f,g,Row means with different superscripts differ significantly. SEM: Standard error of the mean, T1= Basal diet (BD: Negative control) T2 = BD + 1g/Kg diet of Oxytetracycline (Positive control), T3= BD + 2g SB/kg diet, T4= BD + 4g SB/Kg diet, T5 = BD + 2.5g RLM/kg diet, T6= BD + 5.0g RLM/kg diet , T7= BD + 2g SB + 2.5g RLM/kg diet, T8= BD + 2g SB +5.0g RLM/kg diet, T9= 4g SB + 2.5g RLM/kg diet and T10= BD + 4g SB + 5.0g RLM/kg diet.
Table 5: Effect of Sodium butyrate and Rosemary leaf meal on histological traits of samples of duodenum, jejunum and ileum in broiler finisher birds (n=32, N=320)
| Diets | |||||||||||
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | SEM | |
| Finisher | |||||||||||
| Duodenum | |||||||||||
| Villus length (nm) |
1291.5d |
1401. 5c |
1482.1b |
1572.4a |
1387.5c |
1526. 1a |
1372. 6c |
1391. 4c |
1406.2c |
1411.7c |
12.49 |
|
Crypth depth (nm) |
235.16f |
251. 69cde |
261.29b |
283.64a |
258.61bc |
275. 12ab |
246. 25e |
247. 78de |
246.18e |
245.18e |
2.13 |
| Thickness of epithelium (nm) |
58.14d |
78. 02c |
78.19c |
84.68a |
80.09bc |
82. 93ab |
80. 14.bc |
79. 16c |
80.25bc |
80.79bc |
1.15 |
| Thickness of muscularis (nm) |
206.14c |
225. 68b |
228.14b |
240.23a |
221.76b |
240. 56a |
226. 34b |
227. 82b |
219.08b |
224.14b |
1.75 |
| Jejunum | |||||||||||
| Villus length (nm) |
1187.3b |
1275. 1b |
1388.2a |
1405.1a |
1411.2a |
1426. 2a |
1391. 6a |
1388. 5a |
1395.3a |
1405.2a |
14.61 |
| Crypth depth (nm) |
154.76e |
187. 98d |
201.54b |
235.11a |
196.74bcd |
198. 19bc |
188. 64d |
191. 72cd |
190.76cd |
192.49cd |
3.04 |
| Thickness of epithelium (nm) |
47.62e |
52. 49cd |
55.61b |
60.08a |
56.16b |
59. 14a |
52. 26cd |
53. 01bcd |
50.78de |
51.08de |
0.61 |
| Thickness of muscularis (nm) |
137.63c |
157. 75b |
160.82ab |
165.71a |
161.24ab |
164. 25a |
162. 78ab |
163. 25ab |
162.78ab |
161.95ab |
1.33 |
| Ileum | |||||||||||
| Villus length (nm) |
1006.5c |
1204. 7ab |
1197.7ab |
1299.8a |
1107.6bc |
1205. 5ab |
1211. 2ab |
1208. 1ab |
1206.1ab |
1176.6ab |
15.98 |
| Crypth depth (nm) |
156.24f |
171. 08e |
182.62c |
212.55a |
185.64c |
190. 15b |
183. 64c |
175. 64d |
173.14de |
176.64d |
2.26 |
| Thickness of epithelium (nm) |
47.51c |
51. 49bc |
50.17c |
57.29a |
51.66bc |
56. 97ab |
51. 25bc |
50. 97c |
51.58bc |
52.35abc |
0.66 |
| Thickness of muscularis (nm) |
197.81c |
217. 64b |
221.83b |
240.23a |
235.16a |
237. 81a |
218. 61b |
219. 74b |
218.99b |
219.64b |
1.96 |
a,b,c,d,e,f,g,Row means with different superscripts differ significantly. SEM: Standard error of the mean, T1= Basal diet (BD: Negative control) T2 = BD + 1g/Kg diet of Oxytetracycline (Positive control), T3= BD + 2g SB/kg diet, T4= BD + 4g SB/Kg diet, T5 = BD + 2.5g RLM/kg diet, T6= BD + 5.0g RLM/kg diet , T7= BD + 2g SB + 2.5g RLM/kg diet, T8= BD + 2g SB +5.0g RLM/kg diet, T9= 4g SB + 2.5g RLM/kg diet and T10= BD + 4g SB + 5.0g RLM/kg diet.
to have a positive effect in the animal’s intestinal digestive and absorptive capacity. Contrary to the results of the present study, Wu et al. (2018) reported that sodium butyrate or colistin sulphate supplementation did not affect the birds during the starter phase with regards to weight gain, feed intake and feed conversion ratio. They opined that such discrepancies may be due to differences in the age of the birds or health status, feed composition and digestibility, housing conditions, experimental infection models, and the concentration of butyrate in the feed. It has been suggested that the antimicrobial properties and low pH of organic acids inhibit pathogenic intestinal bacteria and decrease the level of toxic bacterial products. As a result, energy and protein digestibility is improved; thereby, the weight gain of broiler chickens is improved (Chacher et al., 2017). Better growth rate in birds’ lifespan might be due to increased well-proportioned micro-flora caused by the presence of butyrate in the broiler ration (Saleh et al., 2018). Also, the dietary inclusion of rosemary leaf meal at 2.5g/kg (T5) had a positive effect on the feed intake and BWG of the broiler birds and also improved the FCR at starter and overall phase of the feeding trial. This result is in line with the findings of Saleh et al. (2018) who reported a positive increase in BWG and performance of broilers at lower inclusion (2.0g/kg) level of rosemary leave meal. They attributed the improvement to the presence of essential oil in rosemary leave meal and its active constituents (phenolic compounds) rich in antibacterial, antifungal and antioxidant activities (Gema et al., 2018). The improvement in growth performance of broiler fed rosemary feed meal might be attributed to stabilization of feed components, improvement in the gut environment, concentration of microflora or by the continuous stimulation of pancreatic and digestive enzymes (Ahsan et al., 2018). Alternatively, the combination of sodium butyrate and rosemary leaf meal had an adverse effect on the feed intake of broiler birds which affected the performance. At the starter phase, feed intake was reduced; this may be due to low palatability in young chicks, although this effect disappeared during finishing phase. This may also be attributed to the capacity of broiler birds to adapt with cellulose content at older age. The reducing gastric pH can stimulate favourable micro-organism and the catabolic enzymes synthesis that can help in the digestion and absorption of amino acids, sugars and fatty acids for an improved performance (Yang and Liao, 2019).
Histological traits of starter and finisher birds
Table 4 and 5 shows the histological traits of broiler birds fed different supplemental levels of sodium butyrate and rosemary leaf meal. Birds fed sodium butyrate and rosemary leaf meal showed significant differences (p<0.05) in all the histological traits measured in both starter and finisher phases. During the starter phase, the highest (p<0.05) villus length, crypth depth, thickness of the epithelium, thickness of muscularis in duodenum, jejunum and ileum was recorded for birds fed T4, although the results were statistically similar with those fed T6 in all of the parameters except for the thickness of the epithelium at the jejunum section of the gut. Apart from birds fed T4 and T6, birds fed T3 also recorded the highest (p<0.05) villus length of the duodenum and jejunum section of the gut, as well as thickness of muscularis in the jejunum part of the gut compared with those fed other treatments. The length of the villus in the ileum section of the gut was highest (p<0.05) for birds fed T2 (1g Oxytetracycline) T4 and T6 while birds fed T1 (Control) recorded the lowest (p<0.05) values of all the histological traits studied.
During the finisher phase, the villus length, crypt depth, thickness of epithelium and muscularis of duodenum, jejunum and ileum were higher (p<0.05) for birds fed T4 and T6 (except for crypth depth of jejunum and ileum) compared with those fed other dietary treatments. Birds fed T1 (negative control) recorded the lowest (p<0.05) histological traits in all the parameters measured. The villus length of the jejunum was highest (p<0.05) for birds fed both single and combined levels of sodium butyrate and rosemary leaf meal, while the value of the villus length was higher (p<0.05) for the ileum section of the intestine in all the treatments, except for those fed the negative control (T1) diet. Figure 1 showed the histomicrograph of the duodenum of birds fed T1 (a), T2 (b), T3 (c), T4 (d), T5 (e), T6 (f), T7 (g), T8 (h), T9 (i), and T10 (j), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis. Figure 2 showed the histomicrograph of the jejunum of birds fed T1 (11), T2 (12), T3 (13), T4 (14), T5 (15), T6 (16), T7 (17), T8 (18), T9 (19), and T10 (20), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis. Again, Figure 3 displayed the histomicrograph of the ileum of birds fed T1 (21), T2 (22), T3 (23), T4 (24), T5 (25), T6 (26), T7 (27), T8 (28), T9 (29), and T10 (30), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis.
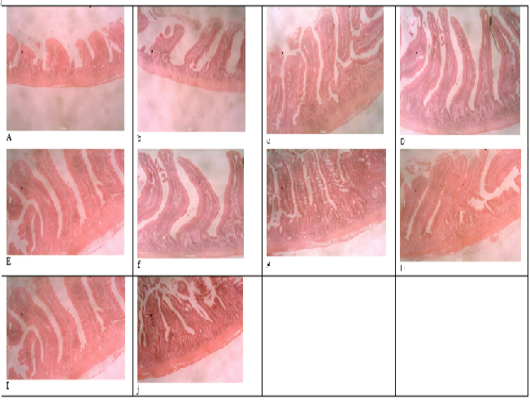
Figure 1: Histomicrograph of the duodenum of birds fed T1 (a), T2 (b), T3 (c), T4 (d), T5 (e), T6 (f), T7 (g), T8 (h), T9 (i), T10 (j), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis.
The gut is the primary site for multitude of processes such as digestion, fermentation, nutrient absorption, nutrient
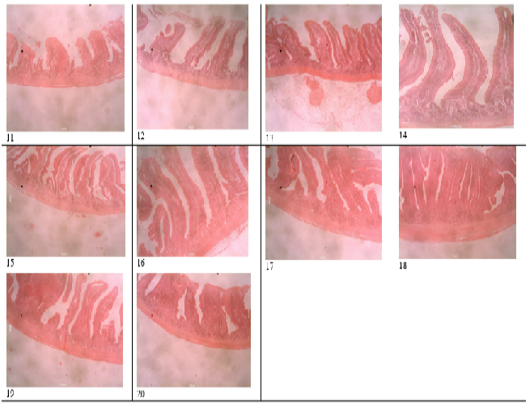
Figure 2: Histomicrograph of the jejunum of birds fed T1 (11), T2 (12), T3 (13), T4 (14), T5 (15), T6 (16), T7 (17), T8 (18), T9 (19), T10 (20), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis.
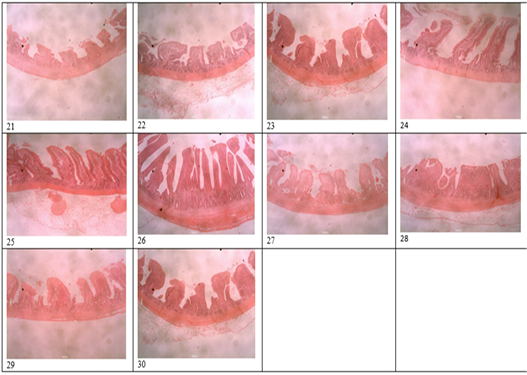
Figure 3: Histomicrograph of the ileum of birds fed T1 (21), T2 (22), T3 (23), T4 (24), T5 (25), T6 (26), T7 (27), T8 (28), T9 (29), T10 (30), showing the villi height, crypt depth, thickness of the epithelium and that of the muscularis.
metabolism, intestinal integrity, immune recognition, immune regulation and development of immune tolerance (Neeraj, 2016). The histology guide is important because it explains the visual art of recognizing the structure of cells and tissues, and understanding how this is determined by their function. It is well documented that microbes can cause a change in the intestinal architecture and the importance of gastrointestinal tract health in poultry has been increasingly documented due to its contributions to their overall health and performance (Alaeldein et al., 2018). Characteristic features of a bird’s digestive tract for the optimal functions include large surface area covered with long healthy villi having shallow crypts. Long villi and shallow crypts provide a larger surface area for the absorption of nutrients and low renewal rate, allowing efficient enzyme production and maturation of the intestinal cells (Abonyi et al., 2020). The villus height and crypt depth in the small intestine are related to nutrient adsorption (Sikandar et al., 2017). Similarly, a higher number of goblet cells per villus indicates higher production of mucins and glycoprotein compounds that that bind with the pathogenic bacteria thus preventing their attachment with the intestinal mucosa (Chacher et al., 2017). The epithelium helps in secretion, selective absorption, protection, transcellular transport and sensing. The intestinal crypt acts as a reservoir of epithelial cells so they are indicative of epithelial cell turnover or renewal rate, while muscularies aid in the propelling of nutrients towards a uniform direction from the lumen to the submucosa (Abonyi et al., 2020). Long villi and shallow crypts provide a larger surface area for the absorption of nutrients and low renewal rate, allowing efficient enzyme production and maturation of the intestinal cells (Ruixia et al., 2020). It is important to note that; any alteration in the diet and the intestinal micro-flora can alter the morphology of gastrointestinal tract of broilers. It will also change the response of the bird’s intestine to dietary changes and may result in either shortening or lengthening of each villus. This means that if the gut health and its functions are damaged, the digestion and absorption of nutrients will be affected (Sugiharto, 2016; Abdelnour et al., 2018; Ruixia et al., 2020). The highest villus length, crypth depth, thickness of the epithelium and thickness of the muscularies of the three sections (duodenum, jejunum, and ileum) of the intestine were recorded for birds fed 4g SB/kg (T4) diet and 5g RM/kg (T6) diets. The results of the present study agrees with the findings of Wu et al. (2018), Ahsan et al. (2016), Abudabos (2018), and Mashael et al. (2020), and they reported in separate studies that the supplementation of sodium butyrate and rosemary leaf meal in the diet improved the villus length and crypt depth in the duodenum and jejunum at different stages of broiler growth. Sodium butyrate accelerates the growth of enterocytes and villus elongation that resulted in the increased villus height and deeper crypts, and this suggests that improved digestive tract maintenance could be the reason for the improved growth performance recorded in this study. The improved digestive tract has a direct stimulating effect on the gastro-intestinal cell proliferation, as reported by Kaunitz and Akiba et al. (2019). Again, the increased villus length and surface area could predict the gain in weight (Arbab et al., 2017). The treatment levels (T4 and T6) used in the present study caused some histological changes in the small intestines by increasing the intestinal surface area, facilitating the nutrient absorption to a greater extent and, thus boosted the growth promoting effect. In addition to bacterial activities, sodium butyrate and rosemary leaf meal have a role in the development of intestinal epithelium, villi growth and also as a major source of enterocytes, which is essential to the health of intestinal mucosa (Ahsan et al., 2016). As sodium butyrate is converted to butyric acid after ingestion, it is preferably absorbed by enterocytes as a source of energy (Andrea and Joshua, 2018).
Contrary to the results of this study, Ali et al. (2017) and (Andrea and Joshua, 2018) reported that the addition of butyric acid glycerides depressed the villus height, villus width, crypt depth and lamina propria thickness of broiler birds. These variable results may be attributed to the fact that uncoated sodium butyrate, with a pKa value being lower than the pH of intestine, is dissociated into ions which cannot be readily absorbed by the enterocytes. Therefore, activities relating to improved intestinal functions are limited only to the upper part of the intestine. However, the fat-coated sodium butyrate may overcome this problem as it is available to the lower parts of the small intestine. In addition, the release of sodium butyrate from the fat covering needs it to be degraded by the activities of lipase enzyme. There are indications that unlike antibiotics, butyrate helps in the maintenance of intestinal villus structure, compared with the negative effects of antibiotics. The observed increase in villus height, crypth depth area, absorptive epithelial cell area and thickness of the muscularis caused by sodium butyrate (T4) and rosemary leaf meal (T6) may indicate an increase in cell proliferation. This improvement in epithelial structure may contribute to the maintenance of the intestinal epithelial integrity by reducing breaks in the mucosal barrier which will restrict the passage of luminal antigens to blood circulation (Andrea and Joshua, 2018; Shaaban et al., 2020). The increase in the villi surface area is significant and can be correlated with an increased proliferation rate of the mucosal cells (Abdelqader and Al-Fataftah, 2016). According to Elnesr et al. (2019), the sodium butyrate action in the improvement of intestinal status may be that butyrate stimulates the intestinal blood flow and the gastrointestinal hormone synthesis. Also, sodium butyrate and rosemary leaf meal increased secretion of peptides which enhanced the proliferation of enterocytes for improved repair of damaged mucosa and increased villi height.
The inclusion of rosemary leaf meal (T6) increased the crypt depth, villus height, thickness of the muscularis and epithelium at the duodenum, jejunum and ileum sections of the chicken gut. This is also the observation made by Saleh et al. (2018) who reported an increased duodenal width of broilers fed 0.5 % rosemary powder and Vit E. The authors went further to explain that the duodenum, jejunum and ileum are main organs responsible for nutrient digestion and absorption. However, the crude fibre content in the rosemary leaf may have cause an expansion seen in the histological traits (Manafi et al., 2016, Ahsan et al., 2018). The jejunum is the main seat of absorption in birds, and the treatments (T4 and T6) used in the present
Table 6: Effect of Sodium butyrate and Rosemary powder on the counts of bacteria in the ileum and caecum (log10 cfu/g) of broiler birds (n=32, N=320)
| T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | SEM | |
| Starter | |||||||||||
| Ileum | |||||||||||
| Lactobacillus |
6.07ef |
8.87d |
10.35b |
11.98a |
10.29b |
11.56a |
9.83c |
9.64c |
9.39c |
9.77c |
0.23 |
|
E.coli |
7.78a |
6.82b |
6.32bc |
5.56cde |
5.31de |
5.02e |
5.97cd |
6.08bcd |
6.01cd |
6.05bcd |
0.14 |
| Salmonella |
7.87a |
6.77b |
5.58c |
5.43c |
5.52c |
5.08c |
5.41c |
5.32c |
5.21c |
5.29c |
0.11 |
| Caecum | |||||||||||
| Lactobacillus |
6.02e |
7.78d |
9.21c |
10.62a |
11.01a |
11.12a |
9.45c |
9.50c |
10.02ab |
10.09ab |
0.24 |
|
E.coli |
6.79a |
5.17b |
5.18b |
4.98b |
4.93b |
4.88b |
4.89b |
4.96b |
4.91b |
5.01b |
0.10 |
| Salmonella |
7.11a |
5.07cde |
5.13cde |
5.54b |
5.44bc |
5.39bc |
4.95de |
5.17bcde |
5.28bcd |
4.88e |
0.10 |
| Finisher | |||||||||||
| Ileum | |||||||||||
| Lactobacillus |
7.11c |
7.97bc |
10.49a |
11.81a |
10.54a |
11.78a |
9.81ab |
9.97ab |
10.09a |
9.88ab |
0.29 |
|
E.coli |
8.55a |
6.72b |
5.81b |
4.91c |
6.19b |
6.24b |
6.31b |
6.41b |
6.19b |
6.35b |
0.16 |
| Salmonella |
7.26a |
6.52ab |
5.68bc |
5.11c |
6.27abc |
5.92bc |
5.11c |
5.54bc |
6.01bc |
6.72ab |
0.14 |
| Caecum | |||||||||||
| Lactobacillus |
7.26f |
8.01e |
12.01ab |
12.82a |
12.06ab |
12.83a |
11.11b |
10.71cd |
10.56cd |
10.38cd |
0.26 |
|
E.coli |
7.92a |
6.67bc |
6.52bc |
6.43c |
6.62bc |
6.39c |
6.76bc |
6.88b |
6.76bc |
6.51bc |
0.07 |
| Salmonella |
8.23a |
6.81bc |
6.62cd |
7.01b |
6.17ef |
6.09ef |
6.33def |
6.13ef |
6.39de |
6.01f |
0.10 |
f, Row means with different superscripts differ significantly. SEM: Standard error of the mean, T1= Basal diet (BD: Negative control) T2 = BD + 1g/Kg diet of Oxytetracycline (Positive control), T3= BD + 2g SB/kg diet, T4= BD + 4g SB/Kg diet, T5 = BD + 2.5g RLM/kg diet, T6= BD + 5.0g RLM/kg diet , T7= BD + 2g SB + 2.5g RLM/kg diet, T8= BD + 2g SB +5.0g RLM/kg diet, T9= 4g SB + 2.5g RLM/kg diet and T10= BD + 4g SB + 5.0g RLM/kg diet.
study may be responsible for the dietary nutrient digestion and assimilation, as they influenced the overall nutritional status, growth and development of chickens. The surface area of the villi determines the absorption activities of the intestines and jejunum in poultry. The histomorphological modulation of the small intestine is held to have a relationship with the production performance of animals.
Gut micro-flora composition of starter and finisher birds
The population of ileum and caecum microbes of broilers fed different dietary levels of sodium butyrate and rosemary leaf meal is presented in Table 6. All the gut micro flora composition traits (Lactobacillus, E.coli, Salmonella) measured in the study for both starter and finisher phases were affected (p<0.05). During the starter phase, the data for Lactobacillus counts of the ileum was highest (p<0.05) for birds fed dietary T4 and T6 compared with those fed other treatments. However, the Lactobacillus counts of the caecum was highest (p<0.05) for birds fed T4, T5 and T6, although they are statistically the same with those that received dietary treatments T9 and T10. Birds fed the negative control (T1) diet had the lowest (p<0.05) Lactobacillus counts in both ileum and caecum parts of the intestine. The highest (p<0.05) E.coli and Salmonella population in both ileum and caecum was seen in birds fed the negative control (T1) diet.
At the end of the finisher phase, the higher (p<0.05) proliferation of Lactobacillus in the ileum section of the gut was recorded for birds fed T3, T4, T5, T6 and T9, though they are statistically the same with birds fed T7, T8 and T10. On the other hand, the highest (p<0.05) Lactobacillus counts in the caecum part of the gut was seen in birds fed T4 and T6, but they are statistically similar with those fed dietary T3 and T5. The population of Lactobacillus was lowest (p<0.05) in both ileum and caecum parts of the gut for birds fed the negative control (T1) diet. The highest (p<0.05) E.coli counts in the ileum was recorded for birds fed the negative control diet (1) compared with other dietary treatments. In the ileum, birds fed T1 had the highest (p<0.05) values for Salmonella, though they are statistically the same with birds fed positive control diet (T2) and T10 diets. The E.coli and Salmonella counts in the caecum were highest (p<0.05) for birds fed the negative control (T1) compared with other dietary treatments.
The micro-flora of the gut has a significant role in animal production and health. They positively affect the hosts’ gastro-intestinal development, digestion, metabolism, pathogen exclusion, immune stimulation and vitamin synthesis (Albazaz and Buyukunal Bal, 2014; Oyeagu et al., 2019). Munyaka et al. (2016) reported that the micro-biota plays an important role in the nutrition and health of the host by promoting digestion and absorption of nutrients, preventing pathogen’s colonization, shaping and maintaining normal mucosal immunity. Bacterial richness and evenness are the major parameters for defining microbial structure and diversity and it is generally expected that dietary manipulations would influence the intestinal microbial structure and diversity (Oyeagu et al., 2019). According to Emily et al. (2019), the major parameters for defining microbial structure and diversity are the richness and evenness of bacterial; and it is generally expected that the manipulation of the diets would influence the intestinal microbial structure and diversity. The intestines of birds have both beneficial bacteria (such as gram-positive Lactobacilli) and potential pathogenic bacteria (Escherichia coli or Salmonella). Generally, the beneficial bacteria represent at least 85% of the total bacteria (Manafi et al., 2016). The bacterial balance between the number of pathogenic bacteria and beneficial bacteria in the intestine is vital for the host, and any negative influence of the intestinal health of chicken often comes from the microbial imbalance in the intestine (Juan et al., 2019). In the present study, sodium butyrate (SB) and rosemary leaf meal (RLM) inclusion (4 g SB/kg diet = T4 and 5 g RLM/kg diet = T6 for starter phase, and 2 g SB/kg diet = T3, 4 g SB/kg diet = T4, 2.5 g RLM/kg diet = T5, and 5 g RLM/kg diet = T6 for finisher phase) increased the population of Lactobacillus spp in both the ileum and caecum sections of the intestine. Lactobacillus have a number of biochemical properties, including the production of antibacterial compounds (Kers et al., 2018) and bile salt hydrolase compounds (Mancabelli et al., 2016; Oyeagu et al., 2019), as well as their probiotics roles that help to maintain gut integrity. The reduction of E.coli and Salmonella was recorded in both ileum and caecum parts of the gut for birds fed T4 and T6 in the starter phase, and T3, T4, T5, and T6 in the finisher phase. This result is in line with the report of Deepa et al. (2017) who observed that amongst the short chain fatty acids, sodium butyrate has the highest bactericidal efficacy against the acid-intolerant species such as E.coli and Salmonella. Also, the antimicrobial activity of phytogenics is attributed to presence of the essential oil in it (Ahsan et al., 2018). Some studies have shown that sodium butyrate and rosemary leaf meal addition could inhibit the proliferation of potentially pathogenic microbial populations in the intestine and improve animal health (Sugiharto, 2016; Saeed et al., 2019). It is possible that, sodium butyrate and rosemary leaf meal supplementation resulted in multiplication of beneficial bacteria in the present study. Hence it can be suggested that butyrate could replace antibiotic totally in practical broiler diets. The increased Lactobacillus and decreased Ecoli and Salmonella from this study could be as a result of sodium butyrate and rosemary leaf meal ability to modulate the intestinal micro-flora balance by controlling the harmful microbial colonization and growth, which stimulates the growth of intestinal absorptive cells, and finally, promoting the growth performance of the birds (Saeed et al., 2019). Sodium butyrate possesses antimicrobial properties due to its important role in the gut and it has a favourable effect on broiler health by controlling the population of pathogenic bacteria and inducing the proliferation of beneficial bacteria by creating an acidic environment in the gut which represents a selective environment that hinders the production of pathogenic bacteria (Abdelqader and Al-Fataftah, 2016; Moquet et al., 2016). According Makled et al. (2019) the sodium butyrate displayed a beneficial impacts on gut micro-biota of meat-type broiler chickens at day 21 through a relative reduction in ileal E. coli and an increase in ileal Lactobacilli count compared with the control. In line with the results of this study, Wafaa et al. (2016) reported that butyrate reduces the invasiveness in Salmonella enteritidis which lead to decrease in caecal colonization. Thus, it can be hypothesized that the effect of sodium butyrate in the distal segments of the gastrointestinal tract could be due to the reduced entry of pathogenic bacteria from the upper parts of gastro-intestinal tract as a compensatory mechanism. The beneficial microbiological and pH-decreasing abilities of sodium butyrate might have resulted in the inhibition of pathogenic bacteria leading to a reduced metabolic need, thereby increasing the availability of nutrients to the host. This also had decreased the level of toxic bacterial metabolites as a result of decreased bacterial fermentation, causing an improvement in the protein and energy digestibility, and facilitating the nutrient absorption to a greater extent as well as improving the weight gain and performance of birds (Ahsan et al., 2018). Wu et al. (2018) did not report any effects of dietary sodium butyrate on E. coli populations in the jejunum in comparison with antibiotic supplemented diets. Similarly, the different concentrations of sodium butyrate in feed did not change E.coli populations in the jejunum (Ahsan, et al., 2016). Likewise, a study showed that coated butyric acid proved to be the best bactericidal agent against Campylobacter jejuni in vitro in comparison with propionic acid, acetic acid and L-lactate (Abudabos et al., 2016). The same effect was observed in the presence of intestinal mucous with a higher dose of sodium butyrate in vitro, however, sodium butyrate supplementation in feed was not effective against pathogenic bacteria in the jejunum (Abudabos et al., 2016). This implied that higher doses of sodium butyrate may be needed to be effective against pathogenic bacteria in jejunum. Another study revealed that sodium butyrate reduced the invasion of Salmonella enterica in intestinal epithelium of broilers due to the regulation of Pathogenicity Island 1 of Salmonella enterica (Wafaa et al., 2016). Similarly, Shang et al. (2018) reported that sodium butyrate favours the growth of Lactobacilli spp. that converts glucose to lactic acid within the intestine of birds, causing the inhibition of pathogenic bacteria such as Salmonella spp and E.coli. Antimicrobial agents, such as sodium butyrate, are known to reduce the levels of intestinal pathogen, which in turn reduces the presence of toxins that causes alterations in the morphology of the intestine (Shaaban et al., 2020). It is evident from reports that the use of rosemary leaf meal has a better effect on the overall performance and immune status of commercial chicks by helping newly hatched birds to develop a favorable and constant intestinal micro-floral population (Chacher et al., 2017). Routinely, butyrate can have direct effects on the intestinal micro-biota in many ways, including the removal of undesired or pathogenic bacteria, manipulation in cell-mediated immune responses through local mucus, enhancement of the antibody rate in the blood, and promotion of epithelial barrier integrity.
Rosemary leaf meals at 5 g/kg (T6) for starter phase and 2.5 g/kg (T5) and 5.0 g/kg (T6) for finisher phase were able to reduce the colonization of E.coli and Salmonella and increased the Lactobacillus counts in the ileum and cecum of the birds at starter and finisher feeding trial. In general, the improvement in growth traits of the birds may be associated with the rosemary leaf meal inclusion levels, capable of changing the enteric flora with a reduced E.coli and Salmonella populations. According to Mancabelli et al. (2016), lowering the gastric pH can stimulate the favourable micro-organisms and the synthesis of catabolic enzymes that helps in the digestion and absorption of amino acids, sugars and fatty acids. In line with the results of the present study, Chacher et al. (2017) reported a positive increase in the Lactobacillus counts for birds fed rosemary meal and yarrow supplementations at day 21 and 42 of age, than the control groups. Once the Lactobacilus spp are established, they might selectively exclude the pathogens from adhering due to their fast colonization, proliferation, and acidification properties in the gastrointestinal tract (Ahsan et al., 2018). The E.coli counts at day 42 also decreased as the rosemary meal supplementation increased compared with the control group. In contrast, Manafi et al. (2016), did not find any effect of natural antibiotics on the Lactobacillus or Coliform counts, and low Coliform counts can be considered as low pathogenic bacteria population which improves the gut health (Chacher et al., 2017). Carvacrol is the essential oil of rosemary plant that has a motivating impact on the propagation of Lactobacillus (Milad et al., 2016). Hydrophobicity is the important aspect of essential oils and their components that enables essential oil partition into lipids in the bacterial cell wall and mitochondria, aiding their distribution. Carvacrol and Rosemary are capable of disintegrating the Gram-negative bacteria outer membrane, releasing lipopolysaccharides, increasing the permeability of the cytoplasmic membrane to ATP, and depolarizing the cytoplasmic membrane (Manafi et al., 2016). Furthermore, it has been proposed that inclusion of oligosaccharides may have a probiotic-like effect through an increase in lactic acid production, thus enhancing the beneficial bacterial proliferation and dropping the presence of pathogenic Gram-negative bacteria.
CONCLUSION
It was concluded that 4 g/kg sodium butyrate, and 5 g/kg rosemary leaf meal supplementation in the chicken diets improved the integrity of the gut and the histological traits, while 2 g/kg sodium butyrate, 4 g/kg sodium butyrate, and 2.5 g/kg rosemary leaf meal inclusions had a better growth traits. Sodium butyrate and rosemary leaf meal inclusion at single levels accelerated the gut health of the birds and these additives can be used successfully as a potent alternative to antibiotics without any deleterious effect on the animal and consumers.
Acknowledgement
The authors wish to acknowledge the Department of Animal Science, University of Nigeria, Nsukka Campus for making available the facilities for this research work. We wish to acknowledge the Centre for Post Graduate Research, Cape Peninsula University of Technology, South Africa for their support.
Authors’ contributions
MCO: Designed the study, collected data, and prepared the manuscript. AOA: Designed and supervised the study. CEO: Designed the study, Co-supervised the study, analyzed the data, prepared and revised manuscript. COO: Analyzed the data and revised manuscript. UO: Handled the microbial laboratory of the study. WIU: Handled the histological laboratory aspect of the study. FBL: Revised manuscript. All the authors read and approved the final manuscript.
Conflict of interest
The authors have declared that no competing interest exists
REFERENCES






