Advances in Animal and Veterinary Sciences
Research Article
Antiviral Effects of Olea europaea Leaves Extract and Interferon-beta on Gene Expression of Newcastle Disease Virus
Rajaa Hindi Salih1*, Shony Mechail Odisho1, Ahmed Majeed Al-Shammari2, Orooba Mohammed Saeed Ibrahim3
1Department of Microbiology, College of Veterinary Medicine, Baghdad University, Iraq; 2Experimental therapy department Iraqi center for cancer and medical genetic research, Al-Mustansiriyah University; 3Department of Physiology and Pharmacology, College of Veterinary Medicine, Baghdad University, Iraq.
Abstract | In this study, we investigated the antiviral activity of Olea europaea (OLE) extracts against Newcastle disease virus (NDV), a highly infectious and economically important poultry virus. For this purpose, viral gene expressions of two structural proteins, matrix (M) and fusion (F) of NDV, were monitoring in chicken fibroblast cells and the impact on virus replication was analyzed using qRT-PCR. Additionally, the virus-restriction activities of IFN-β were analyzed by assessing the expression of viral and host genes. In vitro analysis of the NDV replication revealed that treatment of chicken cells with OLE significantly restricted the NDV replication as was measured by haemagglutination (HA) and titration (TCID50) assays in a pre-optimized OLE-mediated cytotoxicity levels (1000ug/ml). Correspondingly, pretreatment of chicken cells with exogenous IFN-β markedly down-regulated viral gene expression, and this regulation was higher than OLE-mediated virus replication. Furthermore, the impact of OLE on NDV was independent of the IFN-β and was confirmed using ELISA. Taken together, findings of this study propose the utilization of OLE as immunoprophylaxis and exploitation of this function to control the ND in poultry and pet birds.
Keywords | Olea europaea, Antiviral activity, Newcastle disease virus, MTT assay, RT-PCR
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 26, 2017; Accepted | August 13, 2017; Published | October 15, 2017
*Correspondence | Rajaa Hindi Salih, Department of Microbiology, College of Veterinary Medicine, Baghdad University, Iraq; Email: oroobam2000@gmail.com
Citation | Salih RH, Odisho SM, Al-Shammari AM, Ibrahim OMS (2017). Antiviral effects of olea europaea leaves extract and interferon-beta on gene expression of newcastle disease virus. Adv. Anim. Vet. Sci. 5(11): 436-445.
DOI | http://dx.doi.org/10.17582/journal.aavs/2017/5.11.436.445
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Salih et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Newcastle disease (ND) is one of the most important and highly contagious avian diseases caused by virulent strain of avian paramyxovirus serotype 1 (APMIV-1) belonging to the genus Avulavirus within the family Paramyxoviridae. Velogenic strains of NDV cause highly virulent disease and thus is placed under notifiable list A diseases of OIE. The disease carries potential to rapidly spread irrespective of national borders and negatively impact international trade of animals and animal products (OIE, 2005).
The genome of the NDV encoded for at least six proteins; NP, P, L, M, HN and F (Lamb and Kolakofsky, 2001). HN and F proteins are surface glycoproteins and confer vaccine-induced protection. Failure to protect chickens from NDV via vaccine and continuous circulation of the virus in poultry as a result of the presence of genetically and antigenically distinct variant strains that have escaped during the immune response of vaccinated birds (Kilany et al., 2011), beside the emerging of resistance against many of antiviral drugs (Buranathai et al., 2007; Cheung et al., 2006; He et al., 2008). These failed passive immunization demands invention of antiviral agents. Medicinal plants carry main ingredients of medicines in traditional system of health and have been a source of motivation for development of several therapeutic drugs. The use of plants as a source of medicinal value is a relatively old concept because of their advantages, safety, efficacy and availability throughout the world (Ahmed et al., 2002). Medicinal plants have a variety of chemical constituents from natural sources are of interest as possible sources to control viral infection., which have the ability to inhibit the replication cycle of various types of DNA or RNA viruses (Jassim and Naji, 2003). Various research groups in Asia, Far East, Europe and America have given particular attentions to develop antiviral agents from their native traditional medicines plants (Vicente et al., 2005).
These plants are not used as whole rather their specific parts are recommended for the medicinal values in the traditional system (Kumar et al., 2013). Leaves of the important medicinal plant is Olea europaea which has an important place in the Mediterranean diet effects of olive leaves such as the antioxidant, hypoglycemic, antihypertensive, antimicrobial, and antiatherosclerotic have been reported in various studies (Wang et al., 2008). This property can be linked to the fact that the leaves are rich in polyphenols, especially in oleuropein, rutin, verbacoside, apigenin-7-glucoside and luteolin-7-glucoside.
Experimental evidence obtained from human and animal studies demonstrate that phenolic compounds from Olea europaea leaves carry biological activities which may be important in the reduction of risk and severity of certain chronic diseases (Abaza et al., 2015). The chemical composition of olive leaves vary depending on origin, proportion of branches on the tree, storage conditions, climatic conditions, moisture content, and degree of contamination with soil and oils. In addition, structural carbohydrates and nitrogen contents in olive leaves depend on factors such as the variety of the olive tree, climatic conditions, year of produce, and percentage of wood (Molina-Alcaide et al., 2008; Martin-Garcia et al., 2008). The concentration of polyphenolic compounds in olive leaf changes depending on the quality, origin and variety of the plant material (Altiok et al., 2008). Previous results indicate that OLE (olive leaf extract) exhibits antiviral activity against viral haemorrhagic septicemia rhabdovirus (VHSV) and can be considered a potential source of antiviral agents for aquaculture (Micol et al., 2005). In another study, it was revealed that OLE carries anti-HIV-1 activity, and inhibits acute infection and cell-to-cell transmission of HIV-1 (Lee-Huang et al., 2003; Walker, 1996; Kawther Zaher, 2007).
This study was designed to investigate the antiviral potential of OLE against a veterinary pathogen, NDV, which is causing severe economical losses in poultry and current vaccines regimens are insufficient to contain disease in many countries around the globe, including Iraq.
Materials and Methods
Plant Collection
Olea europaea leaves were picked from the local olive trees in Baghdad during June-July season. These leaves were washed under tap water and dried at room temperature (25 - 30°C) for approximately 1-4 days depending upon the moisture contents in leaves. Dried leaves were crushed until converted into fine powder by an electrical grinder and stored in refrigerator until use.
Extraction
Hot aqueous of Olea europaea was prepared as suggested by Qnais et al., 2007. Briefly, a total of 150 g of plant leaves powder was put into the flask, and 3000 ml of boiled distilled water was added with continuous stirring for 15 min, then it was mixed for 15-20 minutes at room temperature. The solution obtained was filtered through a filter paper (twice), the extract was evaporated and finally the remaining material was collected and weighted. The chemical tests were carried out on the plant powder and its aqueous extract by using standard procedures to identify the constituents (Harborne, 1984). Preparation of stock solution of aqueous extract was performed by dissolving 1 gram of plant powder in 10 ml of TDW and sterilized by 0.45 µm syringe (Millipore filter) and stored in -20°C until use.
Viral Isolate
Iraqi virulent isolate of Newcastle disease virus was kindly provided by Experimental Therapy Department, Iraqi Center for Cancer and Medical Genetic Research (ICCMGR). The isolate is named Najaf APMV1/ Chicken/ Iraq-Najaf/ ICCMGR/2012.
Propagation of Newcastle Disease Virus
The NDV was thawed at room temperature and was inoculated in ten days old chicken eggs by allantoic cavity route Cunningham (1973) in a dose of 0.1 ml of virus/egg. Eggs were incubated at 37 °C in a humidified incubator, and checked daily for embryo viability by candling. Embryo mortality after second day were removed from incubator and allantoic fluid was collected and preserved at -80C. The virus was further passaged in SPF (specific pathogen free) embryonated eggs two times to obtain high titer of virus (9-10 HAU/ml) (Alexander, 1998).
Haemagglutination Assay of Newcastle Disease Virus:
The virus was titrated by haemagglutination assay (HA) following the procedure advocated by Cunningham (1973). Two-fold serial dilutions of the virus were made in normal saline beginning with 1:2 dilutions in first well through 1:4096 dilutions in 12th well. A total of 0.5 ml red blood cells (RBCs) suspension (1%) was added in all the wells. The plate was gently mixed by tapping before incubating at room temperature for 45 minutes. HA was determined by tilting the plate and observing the presence or absence of tear shaped streaming of RBCs. The titration was read to the highest dilution of the virus giving complete HA (no streaming) which represented 1HA unit (HAU) (Cunningham, 1973; Beard and Wilkes, 1973).
Preparation of Chicken Embryo Fibroblast (Cef)
The preparation of chicken embryo fibroblast monolayer was performed as described by Hitchner, (1980) and Karel et al. (1998). Briefly, the embryo was taken out in Petri dish containing PBS. The head, limbs, wings, and viscera were removed and discarded. Rest of the embryo was washed using PBS to remove blood. The minced tissue censored to small pieces and transferred into a flask containing 0.25% trypsin solution (pH 7.6-7.8) in PBS for trypsinization for 15 minutes on magnetic stirrer. Cells suspension was filtrated through sterile gauze into sterile beaker, filtered cell suspension was centrifuged at 1500 RPM for 15 minutes and supernatant was discarded. Cells pellets were re-suspended with culture medium (RPMI growth medium at concentration 1ml of packed cells/200 ml of medium) and cells were seeded in 96 well tissue culture plate and incubated at 37°C in an atmosphere of 80-85% humidity and 5% CO2.
Titration of Nd Virus In Cell Culture
Chicken embryo fibroblast cells were seeded at the rate of 1 cells/well in 96 well plates flat bottom. After 24 hours a confluent monolayer was achieved, growth media was discarded and virus suspension was serially tenfold dilution. Then cells were inoculated into 4 wells for each dilution (50µl per well) whereas control cells were inoculated with serum free media. The plate was covered with a sterile adhesive cover and incubated one hour at room temperature to allow virus adsorption. After that, cells were washed with PBS and 200 µl serum free medium was added, the plate was covered again and incubated at 37°C. The inoculated plate was examined daily for five days and NDV titer was calculated on CEF cell as described by Read and Muench, (1938).
In Vitro Cytotoxicity Assay
Cytotoxicity assays were performed according to the method of Karagöz et al. (2007). The cells were inoculated in 96-well plates and were washed with phosphate buffered saline (PBS) before inoculating with and without the OLE extract (2000, 1000, 500, 250, 125, 62.5 μg/mL). After 72 hours incubation, the medium was aspirated and a total of100 mL of MTT solution (5 mg/ mL in PBS, pH 7.2) were added to each well and the plates were incubated for 2 hours at 37 °C. After incubation, 100 mL of dimethyl sul
foxide was added to each well, followed by gentle shaking for 15 min to solubilize the formosan dye. The absorbency was determined on a microplate reader at 492 nm wave length. The assay was performed in triplicate for each of the condition (Chiang et al., 2003). The inhibiting rate of cell growth (the percentage of cytotoxicity was calculated as (IG%) = (A-B)/Ax100, where A is the mean optical density of untreated wells and B is the optical density of treated wells (Gao et al., 2003; Betancur-Galvis et al., 1999).
Antiviral Screening
Tow concentrations of the extract were selected (1000 and 500 μg/ml) and used in antiviral activities screening. The dilution of NDV at the rate of 0.1 MOI (2 TCID50/ml) was used to infect cells in 96 microfiltration plate in a total volume of 100μl whereas the control cell was treated with serum free media only. NDV was added at first for 1 hour at room temperature to allow virus attachment and penetration, virus solution was removed from each well using micropipette and cells were washed with PBS and plant extract with different concentration diluted in warm serum free media (three replicates for each concentration) were added. The plate was covered again with a new sterile adhesive. The plate was incubating at 37°C in CO2 5% incubator for 72 hours (Omilabu et al., 2010). Cell viability was measured by MTT assay and the percentage inhibition was calculated.
Viral- Rna Preparation and Rt-Pcr in One Step
Samples for RT-PCR and ELISA assay:
Tissue culture flasks (25 cm²) containing 1 cells/flask) were incubated at 37°C for 24 hours. The medium was removed from the flasks and flasks were inoculated with NDV at an MOI of 0.01 followed by incubation at room temperature for 1 hour. The plant extract of OLE was added in 4 flasks, and serum free media was added in 4 flasks as virus positive control. The negative control (4 flasks) was treated with serum free media only, and the last 4 flasks were treated with interferon beta (IFN-β) for 15 hours (500IU/ml) before infection with NDV (Krishnamurthy et al., 2006). All flasks were re-incubated at 37°C for 12, 24, 36 and 48 hours (each time contain 4 flasks, one for the NDV, one for olive extract, one for interferon β and one as negative control). In each time above, the cells were harvested with 1 ml of PBS by cell scraper after collected the culture media for detection of interferon β. The suspensions of the cells were collected from each flask into eppendorfs tubes and centrifuged for 10 minutes at 1000 rpm 4°C. The supernatant was discarded and cells pellets were re-suspended in 200 μl RB buffer (supply with the RNA extraction kit) and mixed by vortex, and stored at -80oC till use in qRT-PCR.
Table 1: Primers used in this study
| References |
Sequence |
Gene |
| Al-Habeeb et al., 2013 |
5'- AGTGATGTGCTCGGACCTTC-3' 5'CCTGAGGAGAGGCATTTGCTA-3 |
M gene F R |
| ApE software |
5'-TCCGGCTTAAAGAGAGCATTG-3' 5'ACTGCCACTGATAGTTGTGATAAT3' |
F gene F R |
| Aursnes, et al., 2011 |
5'-TGTGCCGCTAGAGGTGAAATT-3' 5'-TGGCAAATGCTTTCGCTTT-3' |
r RNA 18S F R |
|
Whitmore et al., 2004
|
5'TCCAAGAAAGGACGAACATTCG3' 5'-TGCGGACATCTCCCACGTCAA-3' |
IFN -b F R |
RNA extraction:
Total RNA was extracted from the infected and negative control embryo fibroblast cells which were harvested at each hour post infection (hpi) using the automated extraction RNA kit (Anatolia gene works®, turkey), and each sample was treated with DNase to remove genomic DNA. The concentration of RNA was measured by using the Nanodrop device that can detect the concentration of the RNA in ng/µL and the purities detected by measuring the optical density ratio (OD) at 260/280 nm according to the wave length of absorption of RNA and protein. The accepted purity of RNA samples is between 1.7-1.9 (Sambrook and Russell, 2001).
Quantitative real-time polymerase chain reaction (qRT-PCR):
The primers for RT-PCR were selected from provided references in the Table 1 except for the F gene, which was designed in this study using ApE software (http://biologylabs.utah.edu/jorgensen/wayned/ape/).
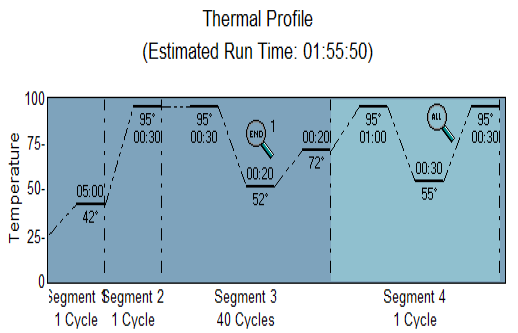
The RT-PCR reactions were carried out according to the procedures of the manufacturers (Agilent Technologies Stratagene, United States). A final volume of 20 uL was prepared using a QuantiFast SYBR Green PCR Master Mix Kit (KAPA Kit) and using a set thermo-profile (Figure 1).
The expression ratio was calculated with a calibrator sample:
Ct according to the following equation: CT (test) = CT gene of interest (target, test) – CT internal control
To compare the transcript levels between different samples the 2 -Ct was used and the CT of gene of interest was normalized to that of internal control gene. The difference in the cycle threshold (Ct) values between the rRNA 18s (internal control gene) and NDV genes (gene of interest) was calculated as the following formula:
CT (test) = CT gene of interest (test) – CT internal control
CT (calibrator) = CT gene of interest (calibrator) – CT internal control.
The calibrator was chosen from the control samples and the CT of the test samples was normalized to the CT of the calibrator:
CT was calculated according to the following equation: CT=CT (test)- CT(calibrator).
Finally, the expression ratio was calculated according to the formula 2-Ct = Normalized expression ratio (Livak and Schmittgen, 2001).
Statistical Analysis
The Statistical Analysis System- SAS (2012) program was used to analysis the parameters. Least significant difference –LSD test was used to compare between means in this study.
RESULTS
Propagation of the ND Virus
The Iraqi strain of NDV (first and second passage) caused 100% mortality rate in chicken embryos in a period of less than 72 hours whereas the third passage killed the chicken embryos at 30-62 hours. Embryos showing 100% mortality (dead embryos within 24 hours) were discarded). Lesions observed on infected dead embryos were congestion and sever hemorrhages as shown in Figure 2 compared to the un-infected control embryos that did not show any congestion or hemorrhage. The virus was purified by centrifugation and quantified by HA which showed positive results as a typical hemagglutination mesh pattern of chicken red blood cell (Table 2).
Table 2: Results of the propagation of NDV in chicken eggs and HA titration
| HA Titration |
Mortality% |
No. of passage |
| 64 | 100% | 1 |
| 128 | 100% | 2 |
| 1024 | 100% |
3 |
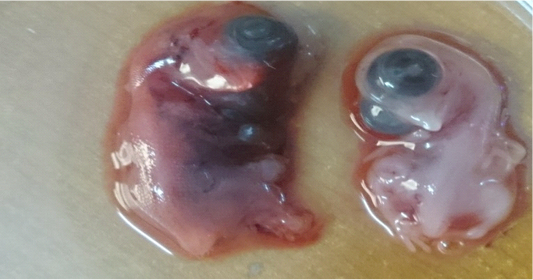
Figure 2: Lesion on dead embryos chicken egg 48hr after inoculated with virulent Newcastle virus (A) infected embryo (B) control
Cytopathic Effects of Nd Virus in Primary Tissue Culture and its Titration
The primary chicken embryo fibroblast cells were used to study the cytopathic effects of NDV from the third passage from chicken embryo eggs and measured the TCID50. CEF cells supported the growth of NDV within 24hours and the cytopathic effects (CPE) were observed as rounding of cells and small cytoplasmic vaculations, which became more obvious by with time until 72 hours of inoculation. These CPEs were characterized by formation of syncytia, granulation, aggregation, rounding, vaculation of infected cell, variable size of plaques, and separation of infected cells in culture media. Corresponding control failed to show any change for the same duration (Figure 3). The titer of the isolate was calculated to be 2 TCID50/ ml.
Extraction of Olea europaea
Extraction of O. europaea leaves (hot aqueous extract) gave a dark green product to Olea leaves which became powder upon drying and yielded percentage of 15.33% which determined according to the equation described by Banso and Adeyemo, (2006):
Percentage Yield =Wt. of plant extract (gm.) / Wt. of plant powder (gm.)
Active compounds in Olea europaea plant leaves, and aqueous crude extract are listed in the Table 3.
Table 3: Phytochemical analysis of Olea europaea leaves extract
|
Result of O. europaea |
Reagent |
Active Compounds |
| (++) Yellow Color | Ethanol+ KOH | Flavonoids |
| (+++) Blue-green color | Ferric chloride 1% | Phenolic Compounds |
|
(+) White ppt. (+) White turbidity |
A- Mayer s reagent B- Tannic acid |
Alkaloids |
| (++) Blue-green color | Ferric chloride | Tannin |
| (++) Thick heavy foam |
Shaking of the extract |
Saponins |
| (++) Red precipitate | Benedict reagent |
Glycosides |
Cytotoxicity of O. europaea
To explore the potential use of OLE as antiviral agent, in-vitro toxicity was performed. The concentration (1000ug /ml) shown the highest percentage inhibition as was determined by the MTT assay. The concentration 1000 and 500 ug/ml were used to determine the antiviral effect of the leave plant extract (against NDV with an 0.1 MOI).
| Conc. (µg/ml) |
Oleaeuropaea |
| 62.5 | 21.23 ± 1.09 |
| 125 | 28.50 ± 1.75 |
| 250 | 37.03 ± 2.82 |
| 500 | 42.91 ± 2.64 |
| 1000 | 49.01 ± 2.73 |
| 2000 | 55.13 ± 2.18 |
| LSD value | 7.259 * |
| (P<0.05). NS: Non-significant | |
Effect of Extract and Ifn- Β on Ndv Gene Expression
One concentration (1000 ug/ml) was used to evaluate the antiviral effects of the plant extract on the expression of the NDV genes in CEF cells, which were successfully detected by qRT-PCR. The virus-infected group showed an increased in fold of M gene expression early in infection at 12hpi and reached at the maximum expression at 36 hpi. Afterward, a decreased expression was noticed at 48 hours. On the other hands, the extract down regulate the viral gene expression at 12, 24, 36 hours and the fold difference from the virus group (control positive) were 0.83, 2.31, 26.78, respectively. At 48 hours, the fold of viral gene increased. The addition of interferon-β significantly reduced gene expression of the virus with time, however at the beginning of the experiment the different in fold gene expression between the virus and IFN-group was not significant. The different became significant at 36hpi (24.86) and at 48 hr. (8.12). The F gene expression was down regulated when the virus was combined with OLE at 24, 36, 48 hpi compared to virus-control with significant difference (P<0.05). The addition of IFN-β down regulated gene expression significantly higher than the extract throughout the period of infection.
Table 5: Percentage inhibition of the OLE on CEF Combination (virus + extract)
|
Combination in CEF cells |
Virus control in CEF cells |
Extract ug / ml |
| 58.7 | 63.8 | 1000 |
| 60.1 | 63.8 | 500 |
Detection of IFN-β by QRT-PCR
Samples which used for the detection of NDV genes were also used for detection of IFN-β which revealed that the viral group stimulate the expression of interferon-β at 12 hours higher than the other groups (IFN-β and Olea extract) which gave (4.14 fold) while other groups gave (3.34 and 3.48), respectively. At 24hpi the expression of this gene in the virus group was upregulated with time to reach 4.46 fold and was downregulated at 36, 48 hours to reach 3.65,2.80 folds and decline to 2.80 at 48 hpi. Gene levels in Olea extract peaked at 12hpi and declined with time of experiment. However, the interferon treated group showed upregulated gene expression at 36hpi, which reached to 3.89 and then downregulated to 2.60 after 48 hpi with ND virus (0.01 MOI) (Figure 3).
IFN- β Quantification by ELISA assay
The supernatant of infected CEF cells were collected after 72 hpi for detection of interferon-β by using ELISA assay. OLE extract had less level of interferon than the virus group (control positive) which had the highest value of IFN- β started at 12hr to 48hr while the interferon group had high level at 12hr and increased at 24, 36 hr but decreased at 48hr.
Discussion
Cytotoxicity of Newcastle Virus
The Iraqi strain of NDV has the ability to kill the chicken embryos from 48 to 72 hours and this indicates the virulence level of the strain which was velogenic type that was able to kill embryos after 40-72hrs (Alexander, 2001; Al-Shammari et al., 2014). These results further confirm that the strain remained virulent after egg passages and hold virulent characteristics. (Singh et al., 2005). The virulent local strain of NDV was previously referred by Zahid and Bunny, (2002) as virulent viscerotropic strain. Hemorrhage was clearly observed in the infected embryos when compared with the control. The virus was purified and then quantified by HA which showed a positive result as a typical HA mesh pattern of chicken red blood cell.
Titration of the Virus
The titer of NDV virus was measured on chicken embryo fibroblast cells for the determination of TCID50, The hallmark of NDV infection in host cells was the formation of syncytia by the isolated virus. It was a typical cytopathic effect caused by the virus which lead to tissue necrosis and might also be a mechanism of virus spread as reported by Qin et al. (2008). Enhanced oncolytic properties as mentioned by (Al-Shammari, 2014; Ebert et al., 2004; Collins and Graham, 2008) was also linked to the syncytia and is considered a conspicuous feature of infection of cell monolayer by NDV. It results from the fusion of infected cells with neighboring infected or uninfected cell. Syncytia may represent a mechanism of viral spread in tissues as mentioned (Freeman et al., 2014). It has been found that the virus yield in normal cells infected with NDV at an MOI of 2 was high after 72 hpi and these results demonstrates that the poor virus yield after low MOI infection of normal cells is due to the limited spread of viral infection (Krishnamurthy etal., 2006).
Aqueous Leave Extract
Hot aqueous leave extract of Olea europaea was used in the current study. Simple drying of fresh leaves at room temperature (25°C) fully preserved oleuropein and verbascoside levels while drying at an elevated temperature of 60°C resulted in losses at various levels of all polyphenols. Boiling of dried leaves is also an efficient method for extracting oleuropein and verbascoside that can give 96% and 94% recoveries of these compounds, respectively (Neradil et al., 2003). Heat-liable component may present in cold aqueous and ethanolic extracts but absent in hot aqueous extracts. Water soluble compounds may present in the aqueous extracts, and water insoluble compounds may present in ethanolic extract. Kamuhabwa et al. (2000) reported that polar compounds, responsible for anticancer activity are water soluble. The colour was graded from dark brown for hot aqueous extract of O.europaea, and extract had a special odour with different intensity. Triantaphyllou et al. (2001) mentioned that aromatic odour indicating that part of essential oil was retained in the extract.
The Cytotoxicity
Measuring of the optical densities (OD) for the stained cells, after treatment with different concentrations of the extract of O. europaea, 1000ug /ml is the IC50, this is in agreement with the finding of Micol et al. (2005) who found that the IC50 after cell treatment of O. europaea extract was 700ug/ml. Zaher, (2007) estimated that the 1000ug/ml were used for antiviral effect and Fulya et al. (2016) demonstrated that the IC50 ug/ml for Olea extract was >1000 ug/ml The cytotoxic effect may be due to the presence of polyphenoles, which have been proved to have anticancer action by many researchers. Owen et al. (2000) demonstrated that antioxidant phenolic compounds present in olive oil are potent inhibitors of free radicals. D’Angelo et al. (2001) and his colleague conducted a recent data on the ability of 3, 4-dihydroxy-phenylethanol (DOPET) to arrest cell proliferation and induce apoptosis in cultured human cells, which support the view that cancer prevention exerted by olive oil could be ascribed to its high content of DOPET and its precursor oleuropein aglycone. Ubied, (2006) demonstrated that the variation in cytotoxic effects of the extract depending on the type, concentration, and duration of exposure. This agree with several in vitro studies which are conducted to assess the cytotoxic effect of different plant extracts and their purified component on various normal and cancer cell lines (Rafiee et al., 2011).
Antiviral Effect of O. europaea Extract
When 1000 ug/ml was used as anti-viral, the results showed that the use of extracts lowering the percentage inhibition of the cells, when the positive control inhibit the cells viability to 63.8, the combination of the extract with virus give 58.7, and 60.1 for the Olea in concentration 1000, and 500ug/ml. However, it is still high which is may be due to cytotoxicity of the extract (Micol et al., 2005).
QRT –PCR
The antiviral effect of the extract investigated by qRT-PCR to study the expression change of NDV mRNA of F and M gene in hour post infection (hpi) (12, 24, 36, 48 hr). The results showed that there was significant differences at 12 hours among groups of the study (virus as positive control, IFN-β and OLE extract) in fold expression of M gene in CEF cells, whereas the expression level of all groups were up-regulated at 24,36 (hpi). However, at 48hpi the M gene expression was up-regulated in the extract which may be due to degradation of active substances. We tested the addition of IFN-β to the cells at 15hours before infection, and observed that there were down regulation in gene expression fold throughout the study period in compare to virus positive control. Richard and Stephen, (2007) revealed that IFN capable of controlling most, if not all, virus infection in the absence of adaptive immunity. However, viruses can still replicate because they have some strategy for at least partially circumventing the IFN response. In addition, the pretreatment of cells with interferon-β can sometimes enhance the IFN yield (an effect called priming) (Honda et al., 2006). The Qu et al. (2013) found that the adding of ChIFN-β (chicken IFN-β) increase the IFN at the beginning of treatment, reaching peak level at 12 hours and then decreased. The positive control clearly had the highest gene expression at 12, 24, 36 hpi of M gene but decline at 48 hpi due to the damage of infected cells as mention by Kumar et al. (2013).
The F gene level of expression in CEF was also estimated and it was observed that the virus has a highest fold at 36 hours followed by declining in comparison with the extract group, while the extract was raised in folds at 48hr, which means the removed or decreased action of it but still lower than the virus group. Yamadda et al. (2009) studied the anti-viral of Olea extract and demonstrated that two phenolic compounds (hydroxytyrosal and oleuropein) lowered the viral titer. Sylvia Lee-Huang et al. (2003) have found that the HIV-1 infection modulates the expression patterns of cellular genes involved in apoptosis, stress, cytokine, protein kinase C, and hedgehog signaling. HIV-1 infection up-regulates the expression of the heat-shock proteins hsp27 and hsp90, the DNA damage inducible transcript 1 gadd45, the p53-binding protein, while it down-regulates the expression of the anti-apoptotic BCL2-associated X protein Bax.
Estimation of Ifn-Β Gene Expression
When estimated the gene expression of the IFN-β in the virus group, it had been observed that the highest fold at 12hr followed by further increase at 24hr. A decline at 36, 48 hr. was observed and the addition of IFN-β did not stimulated the IFN–β gene higher than the virus group and this is due to inhibition of virus replication (Huang et al., 2003), who shown that the V protein of many paramyxoviruses are responsible for blocking the antiviral action of IFN.
Estimated IFN–Β by ELISA Test
A comparable picture was observed in ELISA for detection of IFN-β. There where non-significant differences between the virus and IFN-treated group and also the Olea extract had the lowest concentration.
Conclusions
Many studies have shown that OLE possesses a strong antioxidative activity but this property does not seem directly related to its antiviral effect. The results indicate that O. europaea crude extract can be considered as a potential source of anti-Newcastle virus agent by its effect on viral gene expression. The effects are comparable with IFN-β which is known antiviral cytokine of the host.
Acknowledegements
The authors are thankful to the Department of Microbiology, Physiology and Pharmacology, Baghdad University, for giving consent and support to carry out this study.
Conflict of Interest
There is no conflict of interest.
Authors Contribution
Through the availability of the O. europaea crude extract can be considered as a potential source of anti-Newcastle virus agent by its effect on viral gene expression. it came the idea of a researcher at extracting the active ingredients and study of the effect in preventing Newcastle virus.
REFERENCES