Advances in Animal and Veterinary Sciences
Research Article
Association Between Vitamin D Deficiency and CCL4 Mediated Hepatic Inflammation in Male Albino Rats; Evaluation of Some Biochemical and Antioxidant Marker
Nedaa Ali Eldesouky1, Gehad E. Elshopakey1, Mohamed S. Yusuf2, Fatma M. Abdelhamid1, Engy. F. Risha1*
1Department of Clinical Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, 35516, Egypt; 2Nutrition and Clinical nutrition Department, Faculty of Veterinary Medicine, Suez Canal University, 41522, Ismailia, Egypt.
Abstract | This study conducted to assess the effect of vitamin D deficiency on progression of CCl4 mediated hepatic oxidative stress and inflammation in male albino rat. Forty male albino rats, weighing 120-170g, were randomly divided into four equal groups (n= 10/group):control group, vitamin D deficiency group, CCl4 treated group (1ml CCl4: 1 ml olive oil), at dose 0.2 ml /kg BW once daily and the vitamin D deficiency+CCl4 one group. After six weeks of treatment, biochemical parameters, hepatic oxidative and, antioxidant biomarker, calcium, phosphorus, and Vit D were determined. Our result showed that CCl4+Vit D deficiency and CCl4 treated groups showed significant elevation in the serum ALT, AST and ALP with significant decrease in the total protein and globulin compared with the control group. A significant elevation in total, indirect bilirubin and direct bilirubin level was observed in the CCl4+Vit D deficiency group compared to CCl4 group. Vitamin D level was significantly declined in all experimental groups compared to control group. Moreover, calcium level was decrease in Vit D deficiency group and CCl4+Vit D deficiency group compared to the control. The hepatic antioxidant biomarkers (GSH and SOD) were significantly reduced in CCl4 and CCl4+Vit D deficiency groups, while hepatic MDA level was significantly increased in the same groups unlike the control group. Overall, our findings revealed that the co-existence of vitamin D deficiency might aggravate hepatic inflammation and necrosis; this was confirmed by our biochemical, oxidative stress/antioxidant and histopathological results.
Keywords | Hepatic toxicity, CCl4, Vitamin D deficiency, Oxidative stress, Rats
Received | March 12, 2021; Accepted | March 17, 2021; Published | June 01, 2021
*Correspondence | Engy Risha, Department of Clinical Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, 35516, Egypt; Email: engyrisha@yahoo.com
Citation | Eldesouky NA, Elshopakey GE, Yusuf MS, Abdelhamid FM, Risha EF (2021). Association between vitamin d deficiency and ccl4 mediated hepatic inflammation in male albino rats; evaluation of some biochemical and antioxidant marker. Adv. Anim. Vet. Sci. 9(7): 994-1003.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.994.1003
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Risha. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
CCl4 metabolism is an established model of liver necrosis and fibrosis. The liver damage is created by this metabolism is free radical dependent as CCl4 is oxidized by cytochrome P450 to highly reactive trichloromethyle (CC13) radical that being generated by reductive cleavage of CCl4 bond and generated oxygen radicals and phospholipid peroxides in abundance (Jeong et al., 2020). The generated trichloromethyle free radical causes liver necrosis, destruction of ECM and lipid peroxidation of membranes as well as its cytotoxic effect. The free radical is induced hepatic injury by interacting with unsaturated fatty acids of cell membrane causing lipid peroxidation or cross linking of the unsaturated fatty acids or by covalent binding to important micromoloecules including protein, lipid and nuclear as well as mitochondrial DNA. All of these processes cause damage of cell membrane and leakage of the intracellular molecules (Su et al., 2019).
CCl4 activates several transcription factor including NF-KB which is consider transcription forlorn complex essentially involved in several inflammatory cytokines including IL-6 (Natsume et al., 1999). Upon activation of CCl4, Kupffer cells (resident macrophages) release many chemical mediators such as TNFs, NO, TGF-β1 and IL-6. Also 11SC which are normally quiescent became activated and display a typical acute phase response take a fibroblast like appearance release NO, increased production of typed collagen and thus promote hepatic fibrosis. The TGFf3-1 play a role in the fibrogenic response of HSC to Cc15 and so all grits associated with TGF-β1 singling pathway or inflammatory response pathway were expressed upon CCL4 administration (Chung et al., 2005).
Vitamin D, sunshine hormone, was formed through exposure of skin to ultraviolet rays’ 290_310 nm. Vitamin D is a fat-soluble vitamin that is present in foods. Vitamin D obtained from sun exposure, food, and supplements is biologically inert and must undergo two hydroxylations in the body for activation. First hydroxylation in the liver which converts vitamin D to 25-hydroxyvitamin D3 [25(OH)D3], known as calcidiol. The second is in the kidney and forms the physiologically active 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], which known calcitriol (Nair and Maseeh, 2012).
Vitamin D plays a fundamental role in regulating calcium and phosphorus homeostasis and, in particular, the pathways involved in bone mineralization and bone mass acquisition. Besides these classic skeletal actions, recent studies have demonstrated that vitamin D exerts other significant extra skeletal actions, with a possible role in the pathogenesis of several pathological conditions, including infectious and autoimmune diseases (Saggese et al., 2015). Vitamin D has other roles in the body as modulation of cell growth, neuromuscular and immune function, and decrease inflammation (Holick, 2006). Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D (Nair and Maseeh, 2012). Many cells have vitamin D receptors, and some convert 25(OH) D3 to 1, 25(OH) 2D3. Vitamin D is unique because it can be made in the skin from exposure to sunlight (Autier, 2007). This study aimed to evaluate the effect of vitamin D deficiency and CCl4 on biochemical parameters, besides hepatic oxidative and antioxidant hurt in male albino rat. Moreover, the association between of vitamin D deficiency existence and the severity of liver inflammation were evaluated.
MATERIALS AND METHODS
Experimental Diets
Composition of the control diet and vitamin D free diet were presented in the following Table 1:
Drugs
CCl4 was purchased from El-Gomhoria Co., Mansoura, Egypt. Olive oil imported metro company, Arish city, Egypt.
Animal and Experimental Design
This study was conducted on forty adult albino rats 120-170g. The adults obtained from lab animal unit at Faculty of Veterinary Medicine, Zagazig University. The animal were adapted for lab condition control basal diet and water 2 weeks after adaptation rats were weighted and divided into four equal groups (n= 10/group) as follow:
1. Control group (group I): Rats fed on control diet with orally administration of distilled water.
2. Vitamin D deficiency group (group II): Rats fed on Vit D deficient diet and deprived from sun light for six weeks with orally administration of distilled water.
3. CCl4 group (group III): Rats were treated orally with CCl4 suspended in olive oil (1ml CCl4:1ml olive oil) at dose 1 ml/kg BW/ day for 4 weeks (Abdalla et al., 2013, Osama et al., 2014) and fed on control diet for six weeks.
4. CCl4 +Vitamin D deficiency group (group IV): Rats were treated orally with CCl4 and fed on Vit D deficient diet for six week with deprivation from sun light, with the same previous dose and manner.
Blood and Tissue Samples Collection
Blood samples were collected from each group at the end of the six week. From each rat two blood samples were collected from the medial canthus of the eye. The first blood sample were collected and mixed with anticoagulant (EDTA) for blood counting, while the second sample was collected without anticoagulant and placed in a slant position for twenty minutes at room temperature, then samples were stored in refrigerator for retraction of clot and centrifuged for ten minutes at three thousands r.p.m to separate clear serum samples that carefully transferred to eppendorf tubes to be stored at -20°C until used for biochemical estimation. Also, 0.5 gram of hepatic tissue from each rat was isolated and washed with normal saline then homogenized for determination of liver oxidative stress and antioxidant biomarkers, also specimens from Liver was collected and fixed in 10% formalin for histopathological examination.
Hematological Analysis
Erythrocyte count, hemoglobin (Hb), packed cell volume (PCV), blood indices, leukocytes count were accomplished by manual hemocytometer method using improved
Table 1: Composition of the control diet and vitamin D free diet
| Diet | A | B | Proximate composition | |
| Ingredient | g/kg | g/kg | -ve Vit D diet | +ve Vit D diet (control diet) |
|
Ground Yellow Corn (8.6% CP)a |
200 | 200 | CP% = 17.8 | CP% =17.9 |
| Lactose | 150 | 0.0 | ME= 3690 KCal/kg | ME= 3694 KCal/kg |
| Sucrose | 50 | 50 | ||
| Rice | 0.0 | 165 | Ca = 0.6% | Ca =0. 63% |
|
Yellow peas (25.4% CP) |
180 | 165 | P = 0.35% | P = 0.37% |
| Poultry meal (57.7% CP) | 150 | 135 | Methionine = 0.3% | Methionine = 0.2% |
| Ground alfalfa hay (16.8%CP) | 90 | 100 | ||
| Fine wheat bran (15.9% CP) | 90 | 95 | ||
| Monocalcium phosphate | 10 | 14 | Lysine = 0.2% | Lysine = 0.1% |
|
bVitamin D3 free, Vit.& mineral Premix |
28 | 0.0 | ||
|
cVit.& mineral Premix |
0.0 | 24 | ||
| Salt | 7 | 7 | ||
| Lysine | 5 | 5 | ||
| Linseed oil | 40 |
40 |
||
a Determined values according to AOAC (2000).
b Each 3 kg of vitamin D free vitamins and minerals premix: vit. A 12 mIU, vit. E1000 mg, vit. k3 1g, vit. B1 1g, vit. B2 5g, vit. B6 1.5g, vit. B12 10 mg, biotin 50 mg, pantothenic acid 10g, nicotinic acid30g, folic acid 1g, manganese 60g, zinc 50g, iron 30g, copper 4g, iodine 300 mg, Se 100 mg, cobalt 100mg, and carrier (CaCO3) to 3 kg (MinaKarma care, Egypt).
c Each 3 kg contains the following vitamins and minerals: vit. A 12 mIU, vit. D3 2 mIU, vit. E1000 mg, vit. k3 1000 mg, vit. B1 1000 mg, vit. B2 5000 mg, vit. B6 1500 mg, vit. B12 10 mg, biotin 50 mg, pantothenic acid 10000 mg, nicotinic acid30000 mg, folic acid 1000 mg, manganese 60000 mg, zinc 50000 mg, iron 30000 mg, copper 4000 mg, iodine 300 mg, selenium 100 mg, cobalt 100mg, and carrier (CaCO3) to 3 kg (Golden premix- Selim Pharm Elasher, Egypt).
Neubauer counting chamber. Film were also prepared from each blood sample, fixed and then stained with Gemisa stain for differential leukocytic count (Feldman et al., 2000).
Serum Biochemical Analysis
Alanine aminotransferase (ALT) and aspartate aminotrasferase (AST) were measured with colorimetric kits (Randox, UK). But, alkaline phosphatase (ALP) was estimated with commercial diagnostic kits (Teco diagnostics, USA). The bilirubin was measured by Diamond kits, Meanwhile total protein and albumin were detected by Stanbio Laboratory (USA) kits. While, Glucose, cholesterol, and triglycerides were measured using ready-made kits provided by Spinreact. All parameters were spectrophotometrically detected (5010 photometer, BM Co., Berlin, Germany) according to the enclosed pamphlets. Globulin was calculated by subtraction of serum albumin from total protein and the albumin/globulin (A/G) ratio was calculated by dividing albumin by globulin concentrations (Mahmoud et al., 2020).
Vitamin D 25 hydroxy assay: Total vitamin D is measured by immunoassays (ELISA) by reagents from Calbiotech or IDS or by LC MS MS (PAML, Spokane, WA). Albumin is determined in COBAS Mira plus instrument using bromocresol green. Vitamin D binding protein (DBP) assay: Immunometric assay (sandwich ELISA) using DBP antibody coated microtiter plates and biotin labeled anti-DBP (Bhan et al., 2012). Calcium were determined by quantitative colorimetric method by Spinreact method, described by Young (2001). Phosphorus were determined by according to Tietz (1990) quantitative determination of inorganic phosphorous.
Measurement Of Hepatic Oxidative Stress and Antioxidant Markers
The malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) were determined spectrophotometrically by the enzymatic colorimetric method using Bio-diagnostic kits (Egypt), referring to the manufacturer’s protocols.
Histopathological Studies
Tissue specimen from liver was excised, washed with normal saline and fixed in buffered formalin then, pieces of tissues were sliced at 5micron thickness. The slides for microscopic examination were stained with hematoxylin and eosin as described by (Bancroff et al., 1990).
Statistical Analysis
Results were expressed as the mean ± standard error for five rats in each group. All data analyzed by statistical software
| Parameters | Treatment groups | |||
| Control | Vit D deficiency |
CCl4 |
CCl4+Vit D deficiency |
|
|
RBCs (×106/µl) |
7.94±.069a |
7.54±0.72a |
6.23±0.15b |
5.00±0.06c |
| Hb (g/dl) |
14.90±0.57a |
14.70±0.10a |
8.40±0.10b |
8.46±0.08b |
| PCV (%) |
42.00±1.00a |
40.33±1.33ab |
38.00±0.58b |
38.60±0.52b |
| MCV(fl) |
52.86±0.82c |
53.69±3.58c |
61.04±2.01b |
77.2±1.09a |
| MCH (pg) |
18.76±0.079a |
19.53 ±0.81a |
16.80±0.10b |
13.60 ±0.45c |
| MCHC (%) |
35.51 ±0.74a |
36.51±1.07 a |
21.77 ±0.41b |
22.28 ±0.34b |
|
TLC (×103/µl) |
13.96±0.63b |
12.73±0.94b |
17.15±0.38a |
18.38±.029a |
|
Lymphocyte (×103/µl) |
9.75±0.49a |
9.18 ±0.49a |
5.98±0.36b |
3.63±0.61c |
|
Neutrophil (×103/µl) |
3.02±0.74c |
2.97±0.59c |
10.16±0.32b |
13.88±0.52a |
|
Eosinophil (×103/µl) |
0.25±0.04a |
0.24±0.07a |
0.13±0.07a |
0.35±0.15a |
|
Monocyte (×103/µl) |
0.93±0.36a |
0.34±0.11a |
0.87 ±0.14a |
0.51±0.09a |
RBCs (Red blood cells), Hb (Hemoglobin), PCV (Packed cell volume), MCV (Mean corpuscular volume), MCH (Mean corpuscular hemoglobin), TLC (Total leukocytic count).
Values with different superscript letters consider significance at (p< 0.05).
Table 2: Serum biochemical marker of male albino rat 6th week after induction of Vit D deficiency and liver necrosis (Mean± SE)
| Parameters |
Treatment groups |
|||||||
| Control | Vit D deficiency |
CCl4 |
CCl4+Vit D deficiency |
|||||
| ALT (U/l) |
36.22±1.36b |
|
38.50±0.2b |
54.32±2.87a |
51.25±0.48a |
|||
| AST (U/l) |
50.47±2.17b |
|
51.40±0.62b |
74.00±1.47a |
72.50±1.84a |
|||
| ALP (U/l) |
367.2±23.06b |
|
351.05±34.64b |
438.82±2.40a |
452.23±2.24a |
|||
| Total protein(g/dl) |
6.53±.03a |
|
6.31±0.21a |
5.30±.07b |
5.47±0.03b |
|||
| Albumin (g/dl) |
3.58±0.07a |
|
3.45 ±0.08a |
3.58±0.11a |
3.51 ±0.09a |
|||
| Globulin(g/dl) |
2.94 ±0.08a |
|
2.80±0.28 a |
1.73 ±0.17b |
1.96 ±0.04b |
|||
| A/G ratio |
1.22±0.06b |
|
1.26±0.18b |
2.16±0.31a |
1.79±0.05ab |
|||
| T.bil.(mg/dl) |
0.27±0.01c |
|
0.29 ±0.01c |
0.36±0.18b |
0.48±0.01a |
|||
| Dir.bil.(mg/dl) |
0.13±0.01c |
|
0.14±0.01c |
0.17±0.01b |
0.20±0.01a |
|||
| Indir.bil (mg/dl) |
0.14±0.01c |
|
0.15±0.01c |
0.19±0.01b |
0.27±0.01a |
|||
| Cholesterol (mg/dl) |
74.67±0.88a |
|
67.00±0.58a |
67.00 ±6.43a |
70.50±0.29a |
|||
| Triglycerides (mg/dl) |
135.8±1.6d |
|
141.67±0.88c |
149.70±0.40b |
155.53±1.77a |
|||
| Glucose (mg/dl) |
52.43±0.73d |
|
74.32±1.99c |
79.13±0.09b |
87.25±1.09a |
|||
Values with different superscript letters consider significance at (p< 0.05).
ALT (Alanine aminotransferase), AST (Aspartate aminotransferase), ALP (Alkaline phosphatase), A/G ratio (albumin/globulin ratio), T.bil (total bilirubin), Dir.bil (direct bilirubin), Indir.bil (indirect bilirubin)
program (SPSS for Windows, version 20, USA). ANOVA was used to detect differences between means of all groups using Duncan multiple comparison tests to know significant difference (p<0.05) (Norušis, 2006).
RESULTS
Hematological Analysis
Red blood celld (RBCs), Hb, PCV, and mean corpuscular volume (MCV) were significantly increased in the CCl4 and CCl4+Vit D deficiency groups comparing with the control one. While, mean corpuscular hemoglobin concentration (MCHC) were declined in CCl4 group and CCl4+Vit D deficiency groups in comparison with the control one.
Our results showed a significant elevation in the total leukocytic and neutrophilic counts in the CCl4 group and CCl4+Vit D deficiency group comparing with the control one, while Vit D deficiency group showed no change. Meanwhile, neutrophil showed increase in CCl4+Vit D deficiency group comparing with CCl4 group. As well, lymphocytes was significantly decline in CCl4+Vit D deficiency group and CCl4 group in comparison with the control one.
Serum Biochemical Marker
Our results showed significant elevated in the serum activities of ALT, AST and ALP in the CCl4 group and CCl4 +Vit D deficiency group compared to the control one. CCl4 group and CCl4 +Vit D deficiency group showed a significant decrease in the serum total protein and globulin comparing with the control group, A/G ratio showed significantly increased level in CCl4 group comparing with control group.
A significant elevation in total bilirubin, direct bilirubin, indirect bilirubin were observed in the CCl4+Vit D deficiency group and CCl4 group in comparison with control group, Meanwile, they were insignificantly in Vit D group comparing with control group. Also CCl4+Vit D deficiency showed significantly increased compared with CCl4. Our results clarified that, the serum levels showed a significant increase in glucose and triglyceride levels in all groups when compared with control group or with each other, with the most significant increase was reported in CCl4 +Vit D deficiency group.
Vitamin D, Calcium, and Phosphorus
Vitamins D levels were significantly declined in all experimental groups comparing with control one. Also vitamin D levels were significantly lower in CCl4 +Vit D deficiency group and Vit D deficiency group in compare with the CCl4 group
Calcium level was decreased in Vit D deficiency group and CCl4 +Vit D deficiency group when compared with control group (Figure 1A). The serum phosphorus level in all investigated groups was insignificantly changed.
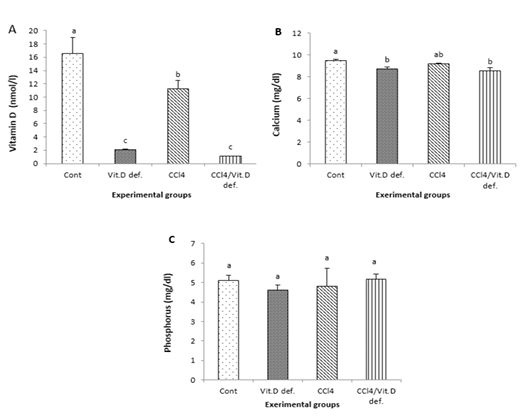
Figure 1: A, Vitamin D; B, Calcium; C, Phosphorus of male albino rat 6th week after induction of Vit D deficiency and liver necrosis (Mean± SE). Values with different superscript letters consider significance at (p< 0.05).
Liver Oxidant and Antioxidant Biomarkers
The antioxidant biomarkers (GSH and SOD) were significantly reduced in CCl4 group and CCl4+Vit D deficiency group comparing with the control one. MDA levels were significantly increased in CCl4 and CCl4+Vit D deficiency groups in comparison with the control one (Figure 2).
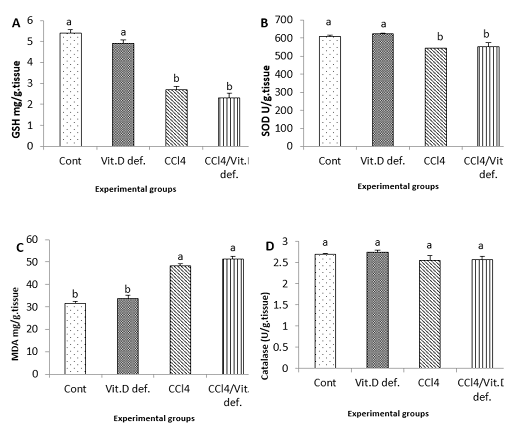
Figure 2: Hepatic levels of A, GSH; B, SOD; C, MDA; D, Catalase of male albino rat 6th week after induction of Vit D deficiency and liver necrosis (Mean± SE). Values with different superscript letters consider significance at (p< 0.05).
Histopathological Results
Liver displayed normal hepatic tissue with normal hepatocytes in control group (Figure 3A). In Vit D deficiency group, liver displayed severe congestion and hemorrhage in hepatic parenchyma (Figure 3B) while in CCl4 group liver displayed scattered necrosis of hepatocytes (Figure 3C). As well, CCl4 and CCl4+Vit D deficiency groups liver displayed round cells infiltration in hepatic tissue and fibroblastic proliferation (Figure 3D).
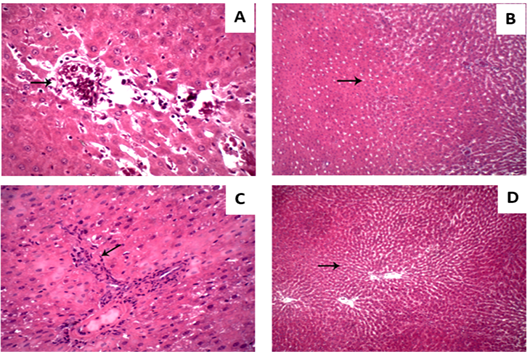
Figure 3: Hepatic micrograph (H&E, x400) of the different experimental groups. (A) control group; Liver displayed normal hepatic tissue with normal hepatocytes. (B) Vit D deficiency group; Liver displayed hemorrhage in hepatic parenchyma. (C) CCl4 group; Liver displayed scattered necrosis of hepatocytes. (D) CCl4+Vit D deficiency group; Liver displayed fibroblastic proliferation.
DISCUSSION
Reactive oxygen species (ROS) due to direct exposure of the extracellular fluid to chemical assaults, coupled with their high oxygen content (Adaramoye and Akinloye, 2000). Also, vitamin D appears to be related to chronic disease prevention and erythropoiesis induction The onset of CCl4-induced liver fibrosis was preceded by RBCs-membrane alterations and the loss of membrane functional integrity. Erythrocytes are at high risk from damage by and differentiation in bone marrow cells (Arabi et al., 2020). It could stimulate erythrocyte precursor cell receptors, which promotes proliferation and maturation of erythroid progenitor cells (Icardi et al., 2013). In adults, a reverse relationship between anemia and vitamin D levels was indicated by several observational studies (Lucisano et al., 2014, Sim et al., 2010, Perlstein et al., 2011, Yoo and Cho, 2015). Also in agreements with our results, Marwah et al. (2012) showed that vitamin D deficiency correlated positively with MCV.
CCl4 administration may gravely alter blood composition including erythrocytes and leukocytes (Abdullah, 2019). Similar findings reported that CCl4 treated animals showed significantly decreased RBCs, hemoglobin, PCV, MCH, MCHC levels and lymphocyte counts and significantly increased MCV levels and total leukocytic and neutrophilic counts (Madthi et al., 2018, Abdullah, 2019). Beside the classic bone metabolism diseases, pathogenesis of several diseases associated with vitamin D deficiency may explained by studying the association between vitamin D, endothelial dysfunction and inflammation (Pludowski et al., 2013). In agreements with our findings, Akbas et al. (2016) support this association and they found that vitamin D deficiency was significantly associated with increased neutrophils and decreased lymphocytes as considered by increased platelet-to- and neutrophil-to- lymphocyte ratios.
In both acute and chronic liver diseases (CLD), hepatic necrosis is a common finding that always is followed by progressive fibrosis with persistence of the underlying cause. Thus during a patient’s clinical evaluation, important information obtained from a liver biopsy is the pattern and extent of necrosis. This necrosis extent is a continuum, ranging from individual cell to massive hepatic necrosis. The pathologist role is to assess necrosis extent and pattern and other morphological changes, with the goal of suggesting one or more possible underlying causes (Krishna, 2017).
From another hand, hepatic disease has been strongly associated with vitamin D deficiency, and the knockout of vitamin D receptor murine model has increased understanding of vitamin D’s role in the liver (Keane et al., 2018). This vitamin deficiency is common in many CLD causes and has been related to the development of non-alcoholic fatty liver disease and chronic hepatitis C infection (Iruzubieta et al., 2014). Despite several impressive in vivo and in vitro studies using animals and human liver cell lines and the ease of access to vitamin D analogues and supplements, clinical data of the patient thus far remains inconclusive. Like other diseases, researches involving vitamin D interventions in hepatic disease have not yielded definitive findings and have tended to be small (Keane et al., 2018).
This study aimed to evaluate the association of vitamin D deficiency existence and the severity of liver inflammation induced by CCl4 in albino rats. To achieve this, we compared between normal controls and rats with induced liver necrosis with and without vitamin D deficiency in biochemical, oxidative stress and antioxidant levels and liver histopathological findings.
In the present study, we used a model of CCl4 for liver necrosis induction. CC14 hepatotoxic effect is related to short lived reactive metabolic intermediates and production of active free radicals under catalyzation through cytochrome P450. Continuous exposure to CCl4 leads to compensatory cell proliferation and liver fibrosis. Consequently, repeated dosing of CCl4 leads to progressively hepatic oxidative stress, injury morphological manifestation characterized by variation in hepatocyte nuclei size and worsening anisonucleosis (Uehara et al., 2014). There are many studies that used CCl4 in liver necrosis or fibrosis induction (Eltahir et al., 2020; Karimi et al., 2020; Wu et al., 2020).
As illustrated in our study, compared with the control group, both CCl4 and CCl4 +vitamin D deficiency groups were associated with significantly elevated activities of ALT, AST, ALP and total bilirubin. CCl4 induced liver damage, serum bilirubin levels have been used to evaluate hepatic injury (Gressner et al., 2007). Bilirubin level was good indicator for pathological manifestation of jaundice, blood joundice caused by break down of red cells, impaired liver function of mechanical obstruction of bile duct (Bishayi et al., 2002). Although albumin was insignificantly changed in all groups compared to the control one, both CCl4 and CCl4+vitamin D deficiency groups showed significantly decreased total protein and globulin. Meanwhile, there were non-significant changes in vitamin D deficiency group.
Liver enzymes (ALT and AST) reflect the status of liver injury and are commonly elevated in patients with liver diseases. In conditions that affect primarily hepatocytes like toxin-induced liver damage and ischemic liver inflammation, marked elevation of these enzymes serum activity was observed. Also, serum bilirubin, albumin and total protein levels offer information regarding liver functional capacity (Hall and Cash, 2012). Beside its role as systemic inflammation monitor, reduced serum albumin might have a role in liver disease aggressiveness (Abdalla et al., 2013). Furthermore, ALP is a hydrolase enzyme present in all tissues entire body, but particularly concentrated in the placenta, bone, bile duct, kidney and liver. ALP is also have impact as poor predictor in liver disease outcome (Yu et al., 2011). Several studies reported that rats treated with CCl4 were associated with changes in biomarkers of hepatic function which characterized by elevated transaminases (AST, ALT), bilirubin and ALP levels and decreased albumin and total protein levels (Xin et al., 2017; Moghazy and Elaidy, 2018).
Because the liver plays an important role in vitamin D pleiotropic functions and metabolism, the question is whether vitamin D deficiency is a contributor to liver dysfunction or a consequence of liver disease (Iruzubieta et al., 2014). However in CLD patients of varying etiologies, this vitamin deficiency has been associated with increased fibrosis severity (Targher et al., 2007). In agreements with our findings, Roth et al. (2012) showed that the existence of vitamin D deficiency affects the progression of liver fibrosis in nonalcoholic fatty liver disease with slightly (non-significant) effect on liver function tests.
Despite, serum phosphorus levels, our findings reported that animal groups with vitamin D insufficiency showed significant decrease in calcium serum levels. Since vitamin D primary function is it required to absorb calcium, then decreased vitamin D levels will causing less calcium absorption. In vitamin D deficiency, it is often claimed that intestinal calcium absorption efficiency is declined (Parfitt et al., 2004; Need et al., 2008). The significance of vitamin D extends is regulating the classical calcium-phosphorus-PTH axis domain (Andress, 2007), The bioactive forms of vitamin D is production in the kidney from inert precursors. So, chronic kidney disease is risk factor for development of vitamin D deficiency (Echida et al., 2012). A reduction in glomerular filtration rates (GFR) limits the delivery of 25(OH)D to the 1-α-hydroxylase enzyme in the proximal renal tubule, and so restricts the ability of the kidney to produce 1.25(OH)2 D (Dusso and Tokumoto, 2011), In chronic kidney disease (CKD), levels of phosphaturic hormone FGF-23 increase, which is response to phosphate retention, which also suppresses production of 1.25(OH)2 D (Liu et al., 2006).
Despite serum cholesterol, our results revealed that CCl4 administration and vitamin D deficiency showed significant increase in blood triglycerides and glucose levels. It was reported that pathogenesis of CCl4-induced hepatocyte injury was associated with the accumulation of triglycerides in hepatocytes and CCl4 increased triglycerides and fatty acids synthesis and the rate of lipid esterification (Boll et al., 2001). Similar elevation in blood glucose was observed by many studies after CCl4 exposure (Khan et al., 2015; Mahmoodzadeh et al., 2017). Many studies reported that vitamin D low levels were associated with high triglycerides, glucose and insulin levels particularly in postmenopausal women (Muñoz-Aguirre et al., 2015; Kwon and Lim, 2016). This vitamin deficiency has been linked to diabetes onset. In accordance with our findings, some studies reported that vitamin D supplementation reduces serum triglycerides levels and may prevent the onset of type 2 diabetes (Sergeev, 2016).
Our results clarified a significant reduction in activity of antioxidant parameters (GSH and SOD) and a significant elevation in the lipid peroxide (MDA) in both CCl4 and CCl4 +Vit D deficiency groups. Oxidative stress is the major cause for several degenerative disorders including hepatopathies. In the liver, cytochrome P450 system bio activation catabolizes CCl4 to highly reactive metabolite, trichloromethyl radical (CCl3-) (Koneri et al., 2008).Further, oxygen reacts with this radical to form the most toxic reactive trichloromethyl peroxyl radical, which can bind to macromolecules and causing cell membrane damage and cell death. Oxidative stress occurred when there is an imbalance between ROS scavenging and production (Vidona and Wadioni, 2018). Thus, it is very clear that CCl4 exposure decrease the activity of natural antioxidants. Also, lipid peroxidation (LPO) is important pathogenic event that damages bio-membranes. It is thought to be an oxidative stress consequence which occurs when the balance between antioxidant and prooxidant mechanism is impaired. CCl4 exposure is associated with increased MDA, reliable LPO marker, in the kidney and the liver (Al-Yahya et al., 2013). Many studies reported such elevation in MDA levels and decreased in antioxidant levels including SOD and GSH after animal treatment with CCl4 (Ritesh et al., 2015; Aleissa et al., 2020).
Increasing data demonstrate that vitamin D has an antioxidant activity (Velimirović et al., 2018). However, in accordance with our results, the role of vitamin D as an antioxidant has not been clearly reported (Afshari et al., 2015). Other studies reported that vitamin D deficiency affects the antioxidant status and its supplementation may be act as enhancer for SOD and catalase activation (Javanbakht et al., 2010). Recently in chronic alcohol-induced mice with liver injury, Hu et al. (2020) found that vitamin D deficiency may aggravates liver inflammation and oxidative stress during.
In contrast to the control group that displayed normal hepatocytes, hepatic tissue, our results showed that vitamin D deficiency displayed severe congestion and hemorrhage in hepatic and renal parenchyma. While in CCl4 group liver displayed scattered necrosis of hepatocytes and kidney displayed renal tubular epithelium vacuolation and severe renal glomeruli congestion and proliferation. As well, coexistence of vitamin D deficiency with liver necrosis aggravates these outcomes and displayed round cells infiltration in hepatic tissue and fibroblastic proliferation and massive hemorrhage replacing renal parenchyma.
Due to their hepatic toxicological effect, many studies reported that CCl4 animals models showed sever hepatic damage that presented by histological findings. For example, Xin et al. (2017) histologically found that the liver lesions of CCl4 -treated animals exhibited necrosis with multifocal distribution and invasive growth and arose within diffuse dysplastic areas. Also, Zayed et al. (2019) found that DEN/ CCl4 co-administration caused liver neoplasia with well differentiated tumor cells, hepatocytic nuclei karyomegaly, oval cells proliferation, sporadic hepatocytes necrosis, hepatocytes cytoplasmic vacuolization and inflammatory infiltration. Similarly, many other studies reported liver specimens pathological and histological alters along with significant collagen fiber content increase induced by these chemicals of showed that CCl4 caused patchy and variable changes in the liver tissue (El Sayed et al., 2019). In human non-alcoholic fatty liver disease (NAFLD), in agreements with our results, Targher et al. (2007) showed that patients with NAFLD have a marked decrease in vitamin D levels which is closely associated with histopathological features of NAFLD.
CONCLUSION
In summary, our findings revealed that the co-excitance of vitamin D deficiency with liver necrosis may cause disease progression and aggravates hepatic fibrosis. This was confirmed by our findings of hematological, biochemical, oxidative stress assessment and histopathologiacal studies. However, our results highlighted the value of vitamin D level measurements and control in liver disease to prevent disease progression.
acknowledgements
All authors are very grateful to all members of the Clinical Pathology Department for their encouragement and support during this study.
CONFLICTS OF INTEREST
The authors declare no conflict of interests
LIST OF ABBREVIATIONS
AST: Aspartate transferase, ALT: Alanine transferase, ALP: Alkaline phosphatase, CAT: Catalase, GSH: Reduced glutathione, MDA: Malondialdehyde, SOD: Superoxide dismutase,
authors contribution
N.A.E.; methodology, formal analysis, data curation, writing original draft, review, and editing. G.E.E., F.M.A., and E.F.R.; conceptualization, validation, visualization, editing final draft and supervision. M.S.Y.; preparation of diet, writing, review and data curation . E.F.R..; prepared the manuscript for publication. All authors read and approved the final manuscript.
REFERENCES






