Advances in Animal and Veterinary Sciences
Research Article
Molecular Detection of Infectious Bronchitis Virus Isolated from Commercial Breeder Farms in Chittagong District, Bangladesh
Md. Inkeyas Uddin1*, Md. Shafiqul Islam2, Tofazzal Md. Rakib2, Shubhagata Das2, Kazi Md. Kamaruddin1, Paritosh Kumar Biswas1
1Poultry Research and Training Center, Chittagong Veterinary and Animal Sciences University, Bangladesh; 2Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Bangladesh.
Abstract | This study was carried out to isolate the infectious bronchitis virus (IBV) in clinical samples from layer and broiler breeder farms in Chittagong district of Bangladesh. The samples were collected from 7 broiler and 3 layer breeder farms experiencing respiratory signs with elevated mortality. The virus was identified from trachea, lung and kidney tissues in clinically infected cases by embryonated egg inoculation and then RT-PCR. From the dead birds, a total 200 organ samples were collected and the prepared inoculums (following standard operation procedure) were injected into 9-11 day old chicken embryo at the rage of 0.2 ml/egg. Total RNA were extracted from collected allantoic fluids that showed characteristic changes in embryo. The results was found that about 34.28% and 28.33% sample positive embryonic changes in broiler and layer breeder farm, respectively. The suspected allantoic fluid was collected and performed for RT-PCR and about 62.5% and 52.94 % samples were found positive in broiler and layer breeder farm, respectively.
Keywords | IBV, Molecular identification, RT-PCR, Gammacoronavirus, Breeder farm
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 05, 2016; Accepted | July 04, 2016; Published | July 09, 2016
*Correspondence | Md. Inkeyas Uddin, Senior Scientific Officer, Poultry Research and Training Center, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong-4202, Bangladesh; Email: sarwarprtc@gmail.com
Citation | Uddin MI, Islam MS, Rakib TM, Das S, Kamaruddin KM, Biswas PK (2016). Molecular detection of infectious bronchitis virus isolated from commercial breeder farms in Chittagong District, Bangladesh. Adv. Anim. Vet. Sci. 4(7): 370-375.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.7.370.375
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Uddin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A wide range of species are affected by Coronaviruses which can cause a diversity of clinical diseases. On the basis of genetic and antigenic mimicry coronaviruses are classified into three genuses. IBV, an avian coronaviruses, belongs to the gamma-coronaviruses group (de Groot et al., 2008). Viral RNA dependent RNA polymerase (RdRp), and Virus specific proteins like structural spike (S) glycoproteins, envelope, membrane or matrix (M) glycoproteins, and nucleocapsid (N) proteins are the proteins encoded by the viral genome. Two crucial spike glycoproteins or peplomers (S1 and S2), projecting from the envelope giving corona conformation, have been reported. In addition, there are numerous accessory and regulatory proteins encoded by the genome (Masters, 2006; de Groot et al., 2008; Jackwood, 2012). IBV, a major poultry pathogen, is most possibly endemic worldwide with concentrated poultry production. All IBV strains can infect a vast range of epithelium of the chicken. They also vary in their capability of replication in non-respiratory tissues. Regarding non-respiratory tissues, the kidney and proventriculus are mostly affected by some strains and produce clinical disease (Han et al., 2011; Resanović, 2015). Urogenital and respiratory mucosas of chickens are common site of replication by vastly contagious virus which can affect all age groups. It is ubiquitous with an incidence of infection approaching 100% but mortality is generally low. 20% to 30% mortalities are stated by different reports and most cases are due to concurrent infections with other opportunistic agents such as E. coli and M. gallisepticum and in permutation with other respiratory viruses like Newcastle Disease Virus, Infectious Laryngotracheitis Virus etc. In breeder chickens, infection can lead to reproductive problems if infected both in young and after maturity. A decline in egg production between 3% and 10%, but reductions of up to 50% has also been observed (Ignjatović and Sapats, 2000; Biswas, 2004). The common serotypes of IBV in North America are Massachusetts (also a protectotype that has ability to cross protect against other serotypes), Arkansas 99, Connecticut IB viruses; Iowa 97, Gray and Holland variants are reported in Europe (Cavanagh and Gelb Jr, 2008). Various serotypes of IBV which are immunologically distinctive and hence vaccine prepared with one serotype cannot shield the other fully (Han et al., 2011). Immunodeficiencies are frequent in commercial poultry due to numerous environmental factors including endemic viruses specifically targeting B and T cells, such as Infectious Bursal Disease Virus, Chicken Infectious Anemia Virus, and Marek’s Disease Virus (Hoerr, 2010; Resanović, 2015). IB concurrent with bursal and thymal depletion of lymphocytes is widespread in commercial chickens (Toro et al., 2006; Resanović, 2015).
IB is seemed to be highly prevalent in semi-scavenging and backyard chickens in Bangladesh. There are insufficient reports regarding the prevalence of IB affecting semi-scavenging as well as backyard chickens in Bangladesh. Infectious Bronchitis Virus in backyard chickens of Bangladesh is first reported by (Alam et al., 2003) by sero-diagnostic approach. Prevalence of 73.6% is stated by (Biswas, 2004) in chickens reared in semi-scavenging system. Based on this high prevalence it is apprehended that the virus plays a significant role in the mortality of semi-scavenging as well as breeder chickens in Bangladesh. This study aims to investigate IBV and confirmation by molecular technique.
Methodology
Study Area and Sampling
This study was designed for detection and molecular identification of Infectious Bronchitis Virus (IBV) in non-vaccinated commercial breeder farms raised under intensive systems of Chittagong, located in south-eastern Bangladesh. Ten farms of Chittagong district were randomly selected; six from northern part and four from southern part. The flocks were not vaccinated against IBV. Samples (trachea, lungs & kidney) were collected from dead chickens after postmortem examination suspected of IB. Samples were subjected to embryonated chicken eggs to isolate IBV for characterization of the virus from 2012 to 2013. For that the study farm was visited regularly. Samples were transported in thermoflax containing ice to Poultry Research and Training Center, CVASU.
Preparation of Virological Inoculums
Virological inoculums for virus isolation was prepared from pooled - homogenized trachea, lung and kidney samples which were collected after post-mortem of suspected dead bird (Figure 1 and 3). The samples were taken into sterile pestle and homogenized with the help of sterile scissors and mortar and added phosphate buffered saline (PBS) (20%) to make a suspension and the suspension were clarified by speed (1000 rpm for 10 minutes) centrifugation. The suspension was treated with penicillin (5 lakh vial per 250 ml of suspension) and a broad spectrum antibiotic Gentamycin (500 µm per ml of suspension).
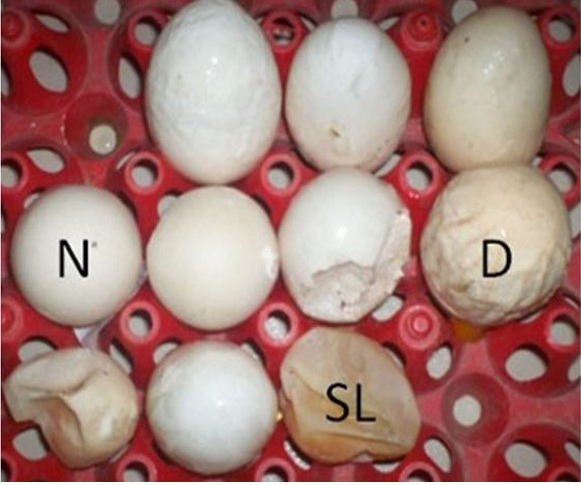
N: Normal Egg; D: Deformed Egg; SL: Shell less Egg
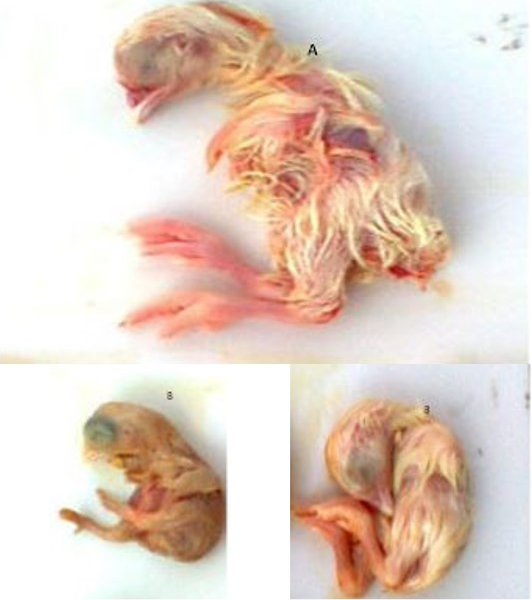
A) Normal Embryo at 18 days of age; B) Dwarfing and curling of embryos at 18 days of age
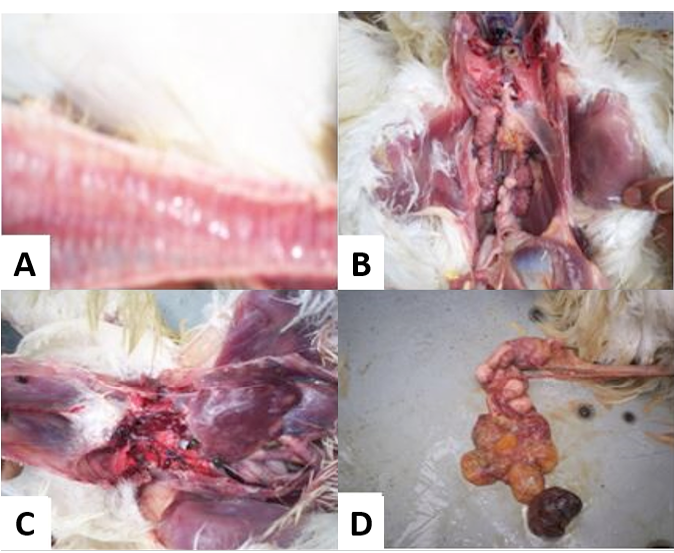
A) Congested trachea; B) Swollen, pale or marbled kidneys with urate deposits in the tubules; C) Visceral gout in chicken with wide spread deposits of uric acid on serosal surface of the body; D) Misshappen ova and oviduct
The suspension was filtrated through 0.45 µl syringe filter and this filtrate is ready for egg inoculation.
Propagation of IBV in Embryonated Eggs
Filtrate (0.2 ml) was injected to two 9-11 days old specific pathogen free (SPF) chicken embryos through allantoic sac route following the technique of (Senne, 1998). After inoculation, the eggs were incubated at 370C for a period of 7 days. During incubation the embryos were examined daily for the development of any gross changes produced by virus in the embryo. The eggs were examined post incubation for two characteristic gross changes of embryos such as curling and dwarfing (Cavanagh and Gelb Jr, 2008). The allantoic fluids were aspirated from the eggs that had such embryonic changes and preserved at -800C.
RNA Extraction
Total RNA was extracted from 300 µl allantoic fluid, collected from incubated 9-10 day old SPF embryonated chicken egg using Viral Gene-spinTM kit (Korea). Extraction was done according to manufacturer’s guideline.
cDNA Synthesis
Viral RNA was first converted into cDNA by the enzyme reverse transcriptase for in vitro amplification by the enzyme polymerase by using Maxim RT PreMix Kit (iNtRON® Biotechnology, Korea). For synthesis of cDNA, 2µl of extracted RNA and 18 µl of distilled water give in to PreMix Kit tube and mixed properly until the pellet was dissolved. The thermal profile of the cDNA synthesis was 45ºC for 1 hour and inactivation of reverse transcriptase 95ºC for 5 min. The cDNA product was stored at -20ºC for next step of PCR.
cDNA Polymerization
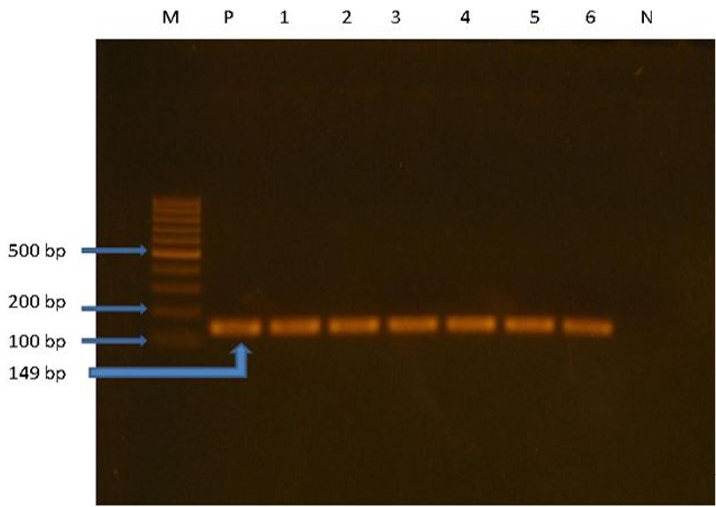
The Polymerase Chain Reaction (PCR) was done in 25 µl volumes, in which the forward primer 1 µl, reverse primer 1µl, 2X master mix 12 µl (Thermo fisher ltd., USA) and nuclease free water 6 µl, cDNA (template) 5µl. PCR was performed in a Thermo cycler (Applied biosystem, 2720 Thermo cycler). The thermal profile comprises initial denaturation for 4 min at 94ºC, then 40 cycles that each having cyclic denaturation for 1 min at 94ºC, annealing for 2 min at 53ºC and extension for 1 min at 70ºC, at last final extension for 10 min at 72ºC. Nuclease free water is used in a negative control instead of template cDNA. Primer used in this study was IBVF 5̍-GCTTTTGAGCCTAGCGTT-3̍ and IBVR 5̍-GCCATGTTGTCACTGTCTATT-3̍. Product Size was 149 bp (Rashid et al., 2009).
Gel-electrophoresis
Gel electrophoresis was done on 1.5 % agarose gel containing syber green dye to analyze RT-PCR product for 30-40 min at 120V potential difference and 90 mA current. The gel was then examined under UV light using an ultraviolet light transilluminator (UV Star, Biometra, Germany).
Data Analysis
Data from laboratory results and questionnaire were analyzed using STATA/IC/13 statistical software. The results were expressed in percentage with 95% confidence interval.
Results
Among 10 selected breeder farms 7 were broiler breeder and 3 were layer breeder farms. Chickens reared in intensive system, with environmentally controlled house, natural floor and high raise cage system of housing. The chicken strains were broiler breeder (Hybro PN, Cobb 500, Hubbard classic) and layer breeder (Saver 579 and Isa brown).
A total of 200 organ samples (140 from broiler breeders and 60 from layer breeders) were collected. During 1st and 2nd passages there were no changes in embryos. However, during 3rd passages the embryos showed stunting, curling, legs compressed upon head and deposition of urates in the kidney (Figure 2). In broiler breeder farms 34.28% and layer breeder farms 28.33% samples were positive for IBV and there was no significant difference between broiler and layer breeder farms in pathology developed during embryo culture (p >0.5).
The suspected positive 65 (Broiler breeder 48 and layer breeder 17) samples were subjected to rt-PCR analysis for confirmation of IB virus. Among them a total of 30 (62.5 %) and 9 (52.94 %) samples were positive for IB virus in broiler and layer breeder farm, respectively (Figure 4).
M: Ladder (100bp); P: Positive control; Line 1, 2, 3, 5 and 6: positive field isolates at 149bp; N: Negative control
Discussion
The study was conducted to know the prevalence of infectious bronchitis virus infection in commercial breeder farms in Chittagong. Infectious bronchitis is of considerable economic worry for poultry producers in Bangladesh (Biswas, 2004) and throughout the world (De Wit, 2000; de Groot et al., 2008). There is a limited study on describing the prevalence of IBV in breeder farm in Bangladesh. Only one report describes the 74% sero-prevalence of IBV in backyard chickens in Bangladesh (Biswas, 2004). In present time, live-attenuated or oil adjuvant killed vaccines are used in vaccination which is the choice for the prevention of IB in chicken. Widely used vaccines to control IB in breeder chickens are attenuated vaccines from HI 20 and 4/19 strains. The disease has considered being stern problem in rapidly growing poultry industry of Bangladesh. Appearance of new variants frequently is also one of the major problems with IBV that most likely cause unsuccessful vaccination. The molecular detection and characterization of new emerging variants are vital to control IBV (Jackwood, 2012; Resanović, 2015).
Infectious Bronchitis Virus grows fine in the developing chicken embryos compared to organ cultures like chicken kidney and trachea (Cook et al., 1976; Ignjatović and Sapats, 2000). Cultivation of IB virus on specific pathogen free 9-11 day old embryonated chicken eggs are methodologically appropriate. No visible changes were observed in first and second passages upon inoculation by intra allantoic route. The characteristic gross changes of dwarfing and embryo mortality were available from third passage. Upon the opening the incubated egg, the embryo appeared curled with malformed feet and compressed over the head, amnion had thickened, yolk sac became shunken, delicate membranes, augmented volume of clear allantoic fluid were the other characteristics lesions found in the inoculated chicken embryos. Our result showed that about 34.28% broiler breeder farm samples were positive for IBV and it is significantly higher than layer farms 28.33%, this difference might be due to breed of poultry.
The observable changes in the embryo during the first egg passage are minimal (De Wit, 2000). Usually embryo mortality and dwarfism are increased from the second passage to onward (De Wit, 2000; Cavanagh and Gelb Jr, 2008). In our study we allowed to grow embryo at least 18 days to see the embryonic changes based on which the presence of IBV could be suspected. We collected allantoic fluid from embryonated eggs those were suspected for IBV and detection by RT-PCR. The embryonic changes caused by the IBV are not pathognomonic. Lentogenic variant of Newcastle disease virus may also develop stunted embryonic lesion in the developing embryos (Han et al., 2011); these changes might also be resulted owing to some other managemental faults and involvement of some other biological agent (Cavanagh and Gelb Jr., 2008). The suspected positive 65 (Broiler breeder 48 and layer breeder 17) samples were subjected to rt-PCR analysis for confirmation of IB virus. Among them a total of 30 (62.5 %) and 9 (52.94 %) samples were positive for IB virus in broiler and layer breeder farm, respectively.
Infectious bronchitis virus can attain severe mortality in chickens if there is concomitant infection by other pathogens such as M. gallisepticum and E. Coli (Nakamura et al., 1996). We didn’t inspect simultaneous infection with opportunistic pathogens. Various reports support the high prevalence of Colibacillosis (21.48%) and Mycoplasmosis (61.74%) in backyard and semi-scavenging chickens in Bangladesh and the elevated prevalence of IB Virus in these chickens has formed the ground of this postulation that some of the chickens might die due to a synergistic relationship between or among IB Virus, M. gallisepticum and E. Coli (Jackwood, 2012).
However, the Reverse transcription-PCR was most sensitive and in our study only suspected positive samples were tested for IB viral infection. The virus isolation is basically applicable in current diagnostic condition of Bangladesh based on their characteristics gross lesions on chicken embryos.
Infectious Bronchitis was prevented principally by using live attenuated virus vaccine such as H120, Ma5, as well as killed vaccine. These vaccines cannot prevent greater than sixty serotypes of IBV which have been reported throughout the world (Ignjatović and Sapats, 2000; Resanović, 2015). Protection given by vaccination with a vaccine of a given serotype is confined mainly against same serotype and fewer against other serotypes. However, it is constructive for advocating control strategy to identify serotype(s) circulating in this area and produce vaccine against circulating serotype(s) of IB virus (Davelaar et al., 1984).
Conclusion
The study was performed aiming to isolation and detection of infectious bronchitis in breeder farms of Chittagong. The present study prove the high prevalence of IBV variants in Bangladesh poultry and it demands the wide spread survey and policy making decision on use of variant strains in vaccine production to get maximum of cross protection against prevailing variants in country. This is necessary to minimize the economic worries to the poultry farms. A thorough epidemiological investigation of the total economic impact of the disease can be achieved by implementing diagnostic techniques such as those in this study. Finally, further studies are recommended focusing molecular detection and characterization of the serotype(s) for adopting suitable vaccination strategy and control measures of the disease in Bangladesh.
Acknowledgements
The author likes to thank all the staffs of Poultry Research and Training Centre (PRTC), Chittagong Veterinary and Animal Sciences University, Bangladesh for their cooperation during the research work. The author is also grateful to the breeder farmers who supported in this study.
Conflict of Interests
There exists no conflict of interest.
Authors’ Contribution
All authors contributed equally.
References