Advances in Animal and Veterinary Sciences
Short Communication
Effect of Intrauterine Administration of Lipopolysaccharide (LPS) on the Transcriptional Profile of IL2, TNFα and IFNγ in Mice
Tumynak Loyi1, Harendra Kumar1*, Sukdeb Nandi2, Manas Kumar Patra1, Rafiqul Islam1, Narayanan Krishnaswamy1
1Division of Animal Reproduction; 2Centre of Animal Disease Research and Diagnosis, Indian Veterinary Research Institute, Izatnagar, 243 122, U.P. India.
Abstract | The effect of intrauterine (IU) infusion of lipopolysaccharide (LPS) on some of the proinflammatory cytokine was studied in a mice model. Female mice of nine week age (n=24) were divided into two equal groups; one group received LPS (E. coli O127:B8) 25μg in 100 μl PBS intrauterine while the other received PBS 100μl as vehicle. Immunokinetic study was done 12 and 24hrs post-LPS treatment by sacrificing 6 mice from each group at each time and relative expression of cytokines (IL2, TNFα and IFNγ) by real time PCR in the uterine tissue. Apparent up-regulation of IL2 cytokine mRNA expression at 12h (2-fold) and 24h (37-fold, P<0.05) was recorded in LPS-infused mice. Similarly, the expression of TNFα mRNA was elevated by 6 and 35-fold at 12 and 24h post infusion, respectively. The increase in the expression of IFNγ was found only at 12h (1.8-fold, P<0.05) in LPS infused mice. It is concluded that intrauterine administration of LPS in the mice elevated the expression of proinflammatory cytokines.
Keywords | Endometritis, Cytokine, Lipopolysaccharide, Mice, Intrauterine
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 07, 2015; Revised | September 08, 2015; Accepted | September 11, 2015; Published | October 21, 2015
*Correspondence | Harendra Kumar, Indian Veterinary Research Institute, India; Email: hkumar1960@gmail.com
Citation | Loyi T, Kumar H, Nandi S, Patra MK, Islam R, Krishnaswamy N (2015). Effect of intrauterine administration of lipopolysaccharide (LPS) on the transcriptional profile of IL2, TNFα and IFNγ in mice. Adv. Anim. Vet. Sci. 3(11): 613-616.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.11.613.616
ISSN (Online) | 2307-8316; ISSN (Print) | >2309-3331
Copyright © 2015 Loyi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Escherichia coli is the most common non-specific, opportunist pathogens that contaminate the postpartum uterus, thereby causing endometritis. The pathogenicity of E. coli is dependent on certain antigens that are essential for adhesion to epithelial cells, motility and cell wall associated endotoxin (like lipopolysaccharide, LPS) (Sheldon et al., 2010). Heat-killed E. coli and LPS initiate similar inflammatory responses by the endometrial cells vis-à-vis expression of inflammatory cytokines and enzymes of prostaglandin synthesis (Herath et al., 2006b), suggesting the importance of LPS in E. coli endometritis. Host cells recognize LPS via surface receptors, leading to initiation of downstream signaling that stimulate the production of nitric oxide, pro-inflammatory cytokines and chemokines (Herath et al., 2006b). These cytokines then further provide a positive feedback loop to increase the immune cell mobilization and further secretion of pro-inflammatory mediators. In addition to LPS, presence of virulent factor fim H gene of E. coli on postpartum day 1-3 was associated with a significantly increased risk of subsequent postpartum endometritis in the cow (Bicalho et al., 2012). A few studies have been carried out in mice with respect to the cytokine profile vis-à-vis the effect of LPS on uterine receptivity and embryonic development (Deb et al., 2005; Aisemberg et al., 2007). When endometrial specific strain of E. coli (cluster 4 O73:H16) and its purified LPS were infused into the uterine lumen of C57BL6 mice, it developed septic metritis with neutrophilic infiltration within 24 h (Sheldon et al., 2010). Recently, Hasan et al. (2013) demonstrated development of endometritis in the mice following intracervical inoculation of E. coli @ 104 cfu/mL after 24 h of treatment. In the present study, the relative expression of selected cytokines in uterine tissue subsequent to intrauterine infusion of E. coli LPS was studied.
Female mice of nine week age (n=24) were divided into two equal groups. One group received LPS (E. coli O127:B8) 25μg in 100 μl PBS intrauterine while the other
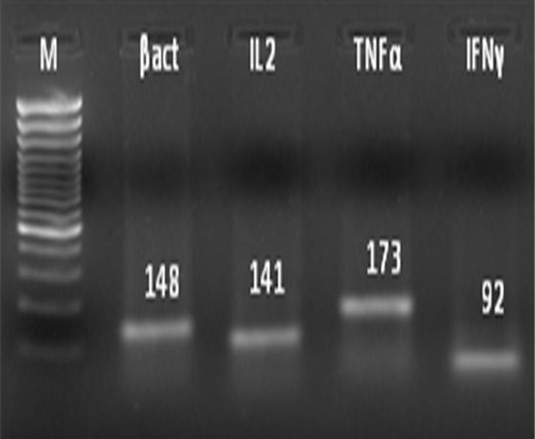
Table 1: Primers used to study the expression profile of genes coding for different cytokines in the mice
|
Gene |
Sequence (5’-3’) |
Primer length (bp) |
Amplicon size (bp) |
|
IL-2 |
F: CCTGAGCAGGATGGAGAATTACA R: TCCAGAACATGCCGCAGAG |
23 19 |
141 |
|
TNF-α |
F: CATCTTCTCAAAATTCGAGTGACA A R:TGGGAGTAGACAAGG TACAACCC |
25 23 |
175 |
|
IFN-α |
F: TCAAGTGGCATAGATGTGGAAGAA R: TGGCTCTGCAGGATTTTCATG |
24 21 |
92 |
|
β-actin |
F:AGAGGGAAATCGTGCGTG AC R:CAATAGTGATGACCTGGCCGT |
20 21 |
148 |
administered PBS 100μl as vehicle. The stage of estrous cycle was determined based on vaginal cytology (Byers et al., 2012). Mice in estrus phase were secured in dorsal recumbency and the LPS or PBS was infused intrauterine. The mice were sacrificed at 12 or 24h interval and the uterine tissue were collected. A total of 1g tissue was weighed and used for total RNA extraction using Trizol reagent following the standard protocol. Extracted RNA was reverse transcribed to synthesize cDNA and polymerase chain reaction was done using specific primer sets (Overbergh et al., 1999) of target genes (Table 1). The PCR products were resolved in 2% (w/v) agarose gel along with a 100 bp DNA marker (Figure 1). The relative expression of IL2, TNFα and IFNγ in the uterine tissue of mice was evaluated by real-time PCR using SYBR green chemistry (Applied Biosystems). β-actin served as endogenous control gene while PBS treated group served as calibrator. The relative fold-change for each cytokine gene at 12 and 24h post-LPS infusion was analyzed by non-parametric Mann-Whitney U test.
In this study, IL2 and IFNγ expression were significantly (P<0.05) up-regulated at 24h and 12h post-LPS infusion, respectively as compared to control. However, a non-significant elevation in expression of TNFα at 12 and 24h post LPS infusion relative to control was recorded (Table 2). A numerical increase in the fold expression of the TNFα without significance might be due to low statistical power consequent to high heterogenity.
Table 2: Real-time transcriptional profile of the cytokine genes in the uterine tissue of LPS- infused mice relative to control
|
Cytokine gene |
Time Post-LPS infusion (h) |
Fold increase (n=6) |
P value |
|
IL2 |
12 |
2.38±1.36 |
0.647 |
|
24 |
36.75±23.48* |
0.037 |
|
|
TNFα |
12 |
6.258±3.00 |
0.647 |
|
24 |
35.14±17.64 |
0.431 |
|
|
IFNγ |
12 |
1.81±0.02* |
0.022 |
|
24 |
0.98±0.49 |
0.115 |
* Significance at 95% level (P<0.05)
LPS is the main pathogenic ligand of E. coli and a strong activator of innate immune response (Herath et al., 2006a). Following infection, an immune response is initiated by cell surface Toll-like receptor-4 (TLR4), CD14 and MD2 (Herath et al., 2006b; Herath et al., 2009), that detect pathogens and in turn trigger signaling pathways that activate molecules (transcription factors) that controls the induction of a proinflammatory immune response through activation of genes encoding cytokines (IFNγ, IL2 and TNFα) and chemokines (Bonizzi and Karin, 2004). It is suggested that LPS can trigger TNFα release by interferon-primed macrophages and induce IFNγ production, resulting in an additive effect on the production of TNFα. In addition, a higher endometrial expression of TLR4 in clinical endometritis was reported in buffaloes (Loyi et al., 2013). It is reported that treatment of bovine granulose cells in culture with LPS adversely affected oocyte maturation and increased IL6 transcripts (Bromfield and Sheldon, 2011) and steroidogenic capacity through TLR4 pathway (Price et al., 2013).
The elevated levels of TNFα and IFNγ in our study are in agreement with the reports of Nandi et al. (2010) who reported that bacterial LPS in the bloodstream induced production of inflammatory cytokines such as TNFα, IL1β and IFNγ. TNF is secreted from a variety of gestational cells upon stimulation with bacteria or lipopolysaccharides in vitro including placental fragments, amnion, and chorio-decidua (Sato et al., 2003). Herath et al. (2006b) also described the increased expression of TNFα in the cultured endometrial epithelial and stromal cells stimulated in vitro with LPS. However, Yang et al. (2009) did not observe a classical predominance of Th1 cytokine in the plasma of mouse of pre-term labour following intrauterine infusion of LPS.
Upregulation of IL2, IFNγ and TNFα transcripts in the uterine tissue of mice following LPS infusion in the present study indicates uterine inflammation. Intravaginal inoculation of mice with specific bovine uterine isolates of E. coli will further support the present observation. The efficacy of novel intrauterine drugs for the treatment of endometritis in the bovine may be tested using this model.
Acknowledgments
The authors are grateful to the Director, ICAR - Indian Veterinary Research Institute, Izatnagar for providing the facility to carry out the research work.
Conflict of interest
There is no conflict of interest.
AUTHORS CONTRIBUTION
The work was carried out by Tumnyak Loyi as part of her PhD thesis under the guidance of Harendra Kumar and Sukdeb Nandi. Manas Kumar Patra and Rafiqul Islam assisted in designing of experiment, sample collection, and analysis of result. Krishnaswamy Narayanan assisted in statistical analysis and preparation of the manuscript.
REFERENCES