Advances in Animal and Veterinary Sciences
Reaserch Article
Semen Evaluation of Arab Stallions during Steps of the Freezing–Thawing Protocol
Najjar Amel1, Ben Said Samia2, Kalamoun Soumaya2, Benaoun Belgacem3, Ben Haj Aissa Sonia1, Ezzaouia Mohamed3
1National Institute of Agronomy of Tunisia; 2High School of Agricultural of Kef, Tunisia; 3National Stud Farm, Sidi Thabet, Tunisia.
Abstract | The aim of the current study was to determine the influence of steps involved in the freezing–thawing protocol on semen quality of Arab stallions. A total of 48 ejaculates were collected (step 1) and undergone the first dilution in INRA96® at +37°C, followed by incubation at +22°C (step 2). A second dilution with INRA Freeze® was carried out after centrifugation and supernatant removal (step 3). The diluted semen was kept at +4°C, then packaged in straws of 0.5 ml (step 4) and frozen into liquid nitrogen at – 196°C. After 48 hours of storage, the semen was thawed (step 5). The percentages of motile sperm (%MS), abnormal head (%AH), mid-piece (%AM), flagella (%AF), cytoplasmic droplets (%CD) and abnormal sperm (%TAS) were studied at each step. Results showed that the %MS and %TAS were higher in steps 1, 2, 3 and 4 as compared to those of step 5 (p<0.05). The %AM was higher in step 5 as compared to that of the other steps (p<0.1). However, the %CD and %AF were higher respectively in the step 3 (p<0.01) and step 4 (p<0.05) than those of the other steps. Consequently, the semen quality of Arab stallions was affected during the freezing–thawing protocol. So, it is recommended to study the contribution of molecules in extenders such as vitamin C or glutamine to improve sperm motility and avoid increasing abnormal sperm during the freezing-thawing protocol.
Keywords | semen quality, freezing, thawing, steps, Arab stallions
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 09, 2016; Accepted | June 11, 2016; Published | June 08, 2016
*Correspondence | Najjar Amel, National Institute of Agronomy of Tunisia; Email: amelnajarbenmatoug@gmail.com
Citation | Amel N, Samia BS, Soumaya K, Belgacem B, Sonia BHA, Mohamed E (2016). Semen evaluation of Arab stallions during steps of the freezing–thawing protocol. Adv. Anim. Vet. Sci. 4(6): 279-282.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.6.279.282
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Amel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The development of artificial insemination with frozen – thawed semen in horses requires efforts to improve the quality of frozen sperm and minimize the inter-individual variability among stallions during the freezing process (Loomis and Graham, 2008). In fact, during the stages of the semen freezing –thawing spermatozoa endure cellular damages, especially for plasma membrane that causes physiological and functional modifications (Pena et al., 2003). Morris et al. (2007) reported that toxicity and osmotic stress could be caused by the use of cry protectants and extenders. Moreover, Watson (2000) reported that oxidative reaction and premature ageing occur during the cooling stage of the freezing process and contribute to sperm death. Vidament (2005) showed that there are stallions known for their high fertility in natural mate but their semen cannot support the stages of the freezing and thawing process. Therefore, this study was designed to determine the semen quality of Tunisian Arab stallions during the steps of the freezing – thawing protocol.
Material and methods
General
Semen was collected twice weekly from 6 Tunisian Arab stallions (mean age = 13±2 years) using a Missouri artificial vagina (IMV, France). Semen collection took place at the semen production centre of the National stud farm of Sidi Thabet, in the north of Tunisia.
Freezing and Thawing Process
A total of 48 fresh ejaculates were collected (step 1) and then frozen. During the freezing process, semen was firstly diluted in INRA96® (200 ml, IMV, L’aigle, France) at +37°C (¼ semen, ¾ extender), and put for 10 minutes in a water bath at +22°C (step 2). Then, semen was centrifuged at 600g for 10 minutes. The supernatant was eliminated and the sperm pellet was diluted again in INRA Freeze® (200 ml, IMV, L’aigle, France) (step 3 = after the equilibration stage). The diluted semen was kept at +4°C about 80 minutes. Then, semen was identified and packaged in straws of 0.5 ml (step 4 = after the cooled stage). After that, straws were frozen in a programmable cell freezer before being plunged into liquid nitrogen at – 196°C. After 48 hours of storage, 2 straws per ejaculate were thawed for quality control (step 5 = after thawing): Semen was thawed in a water bath at +37°C during 30s and diluted with 2ml of a skimmed milk.
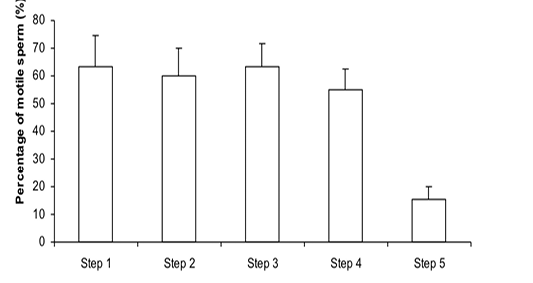
Figure 1: Percentage of motile sperm (%MS) during steps of the freezing-thawing semen protocol (means ± sem); a,b: p<0.05
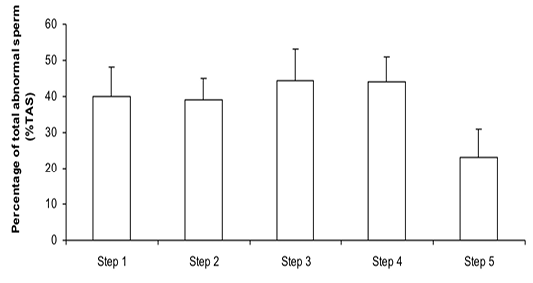
Figure 2:Percentage of total abnormal sperm (%TAS) during steps of the freezing-thawing semen protocol (means ± sem); a,b: p<0.05
Semen Evaluation
Semen evaluation was performed following each step of the freezing and thawing protocol (step 1 to step 5). A subject appraisal of sperm motility (percentage of motile spem: %MS) was achieved by optic microscopy, identically used by Rousset et al. (1987). Sperm morphology was assessed using a phase-contrast microscope (Jasko, 1992). The percentages of abnormal head (%AH), mid-piece (%AM), flagella (%AF), cytoplasmic droplets (%CD) and the percentage of total abnormal sperm (%TAS) were determined after eosin-nigrosin staining (Haras Nationaux, 2004).
Statistical Analysis
The statistical analysis was developed on software SAS (SAS, Institute, Inc). ANOVA was carried out to compare variables between the steps of the freezing and thawing process. The threshold of significance was 5%.
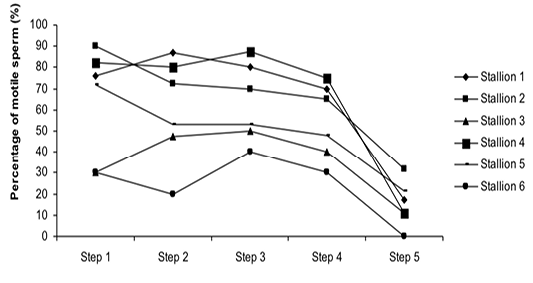
Figure 3: Variation of the percentage of motile sperm (%MS) according to stallions during steps of the freezing-thawing semen protocol
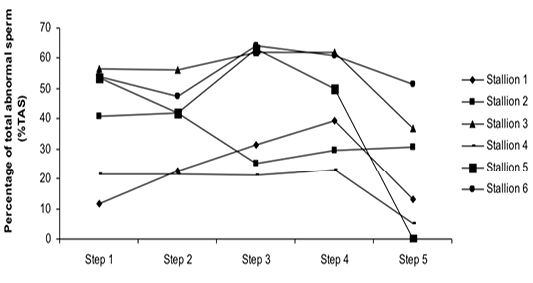
Figure 4: Variation of the percentage of total abnormal sperm (%TAS) according to stallions during steps of the freezing-thawing semen protocol
Results
The %MS (Figure 1) and %TAS (Figure 2) were higher in steps 1, 2, 3 and 4 as compared to those of step 5 of the freezing-thawing semen process (For %MS: respectively 63.5, 60, 63.5 and 55% vs 15.5%, p<0.05; For %TAS: respectively 40, 39, 44.5 and 44% vs 23%, p<0.05). The statistical analysis showed that %MS and %TAS varied among stallions during the freezing – thawing protocol (p<0.05). It decreased mostly from step 3 for the %MS (Figure 3) and step 4 for the %TAS to reach the lowest values at step 5 after semen-thawing (Figure 4).
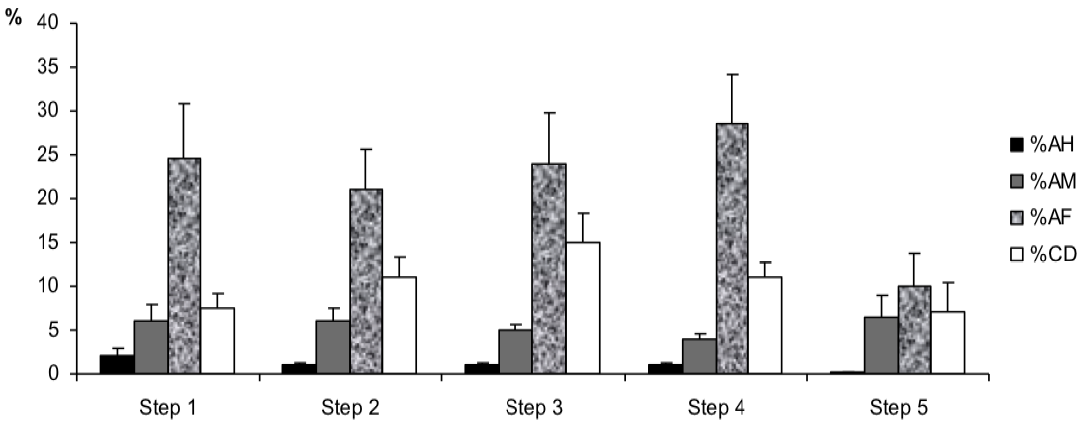
However, for sperm morphology features (Figure 5) results showed that the percentage of abnormal mid-piece (%AM) was higher in step 5 in the post-thawing semen as compared to the one of the other steps of the freezing-thawing semen process (7% vs 6, 6, 5 and 4%, p<0.1). Besides, the percentages of cytoplasmic droplets (%CD) and abnormal flagella (%AF) were higher respectively in step 3 and step 4 (For %CD: 15% vs 7.5, 11, 11 and 7%, p<0.01; for %AF: 28.5% vs 24.5, 21, 24 and 10%, p<0.05).
Discussion
The freezing-thawing semen process affected the semen quality as reported by previous studies. England and Pronzio (1996) and Bedford et al. (2000) found out adverse damages in all semen parameters quality such as mobility, viability, membrane integrity and morphology may occur. Rota et al. (1999) showed that freezing-thawing increases the intra cellular calcium concentration and changes structural and functional modifications that lead to sperm permeability. In our study, sperm mobility decreased significantly in the last step after thawing the semen. This finding coincides with the results of Nield et al. (2003). In fact, they found out that the most pronounced damage to membranes and the greatest decreased progressive motility occurred in samples of all stallions after thawing. Gibb and Aitken (2016) reported that sperm damages could be attributed to changes in the metabolic activity of the cell as the cold-shock, caused by lipid membrane separation.
The changes on sperm morphology during cryopreservation processing were confirmed in several studies (Ruzul et al., 2001; O’Connell et al., 2002; Ahmed et al., 2014). But in our case, results showed an unjustified decrease in the %TAS after the thawing. This could be attributed to material or manipulation conditions in the laboratory. However, our results showed that the %CD and %AF were significantly higher respectively in step 3 after the equilibration stage and step 4 after the cooled stage. Consequently, it could be deduced that the equilibration and the cooled stages of the freezing process increase a deleterious effect on the sperm morphology. Indeed, the sperm morphology changes can be a result of damage to mitochondria activity, acrosome and sperm tail (Wooley and Richardson, 1978; Holt, 1997). The osmotic stress is the main cause of morphologic sperm damage between the intra and extra cell medium (Mazur and Cole, 1989; Pegg, 2007).
In conclusion, the semen quality of Arab stallions was affected during the freezing – thawing protocol. The sperm mobility decreased after the thawing step (step 5), but the sperm morphology increased after the equilibration (step 3), cooling (step 4) and the thawing (step 5). It will be interesting to study the contribution of molecules in extenders such as vitamin C or glutamine to improve sperm motility and avoid increasing abnormal sperm during the freezing-thawing protocol.
Acknowledgments
The study was financed by the National Stud Farm of Sidi Thabet.
Conflict of Interests
There exists no conflict of interest.
Authors’ Contribution
Najjar Amel participated to the experiment, analysed the data and wrote the article. Ben Saïd Samia and Kalamoun Soumaya did the experiment. Benaoun Belgacem was the co-supervisor. Bel Haj Aissa Sonia corrected the langage and Ezzaouia Mohamed was the supervisor.
References