Advances in Animal and Veterinary Sciences
Research Article
Nigella sativa Seed Extract Affected Tegument and Internal Organs of Trematode Paramphistomum cervi
Muhammad Hambal1*, Henni Vanda2, Siti Rani Ayuti3
1Veterinary Parasitology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala 23111, Indonesia; 2Veterinary Pharmacology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala 23111, Indonesia; 3Veterinary Biochemistry Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala 23111, Indonesia.
Abstract | Paramphistomum cervi infects ruminants and wildlife with significant economic losses. Anthelmintic resistance and high cost of treatment could be resolved by using traditional herbs to replace synthetic drugs. Nigella sativa is one of the alternative plants to treat paramphistomiasis. This study aimed to investigate the efficacy of N. sativa seed extract towards adult Paramphistomum flukes in vitro. The flukes were divided into six groups, consisted of ten flukes each, and soaked in 5% (T1), 10% (T2), 25% (T3), 40% (T4) N. sativa extract, in PBS (C0) as the negative control, and also in albendazole (C1) as the positive control. The motility and mortality time of flukes were recorded, and the dead flukes were further prepared for histopathology observation. The results revealed that treatment and control groups were significantly different (p<0.05). The most effective concentration was 40% N. sativa (T4), followed by T3, T2, and T1. N. sativa extract had stronger effect than albendazole towards P. cervi in vitro. In term of histological findings, the flukes in T3 and T4 showed disintegration and breaking of tegument, and necrose of parenchyma cells, while in T1 and T2, the tegument was still intact. Reproductive organs were also affected by N. sativa extract. We concluded that 25% and 40% of N. sativa seed extract showed good anthelmintic property. The results suggested the potential of. N. sativa extract for advancing plant-based anthelmintics to control P. cervi.
Keywords | Anthelmintic property, Nigella sativa, Paramphistomum cervi, histopathological changes, in vitro.
Received | March 12, 2021; Accepted | March 17, 2021; Published | June 01, 2021
*Correspondence | Muhammad Hambal, Veterinary Parasitology Department, Faculty of Veterinary Medicine, Universitas Syiah Kuala 23111, Indonesia; Email: hambal.m@unsyiah.ac.id
Citation | Hambal M, Vanda H, Ayuti SR (2021). Nigella sativa seed extract affected tegument and internal organs of trematode paramphistomum cervi. Adv. Anim. Vet. Sci. 9(7): 978-982.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.978.982
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hambal et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Trematode Paramphistomum cervi causes paramphistomiasis in cattle, goats, sheep, and other ruminants, including wildlife. This fluke is usually found in rumen and abomasum, and triggers annual economic loss up to US $ 3 billion (Elelu et al., 2018; Hambal et al., 2020). Paramphistomiasis usually have no significant clinical symptoms, in chronic infection, it will cause severe weight loss, low milk production, infertility, and even death (Bilal et al., 2009; Ozdal et al., 2010).
Synthetic Anthelmintic have been used for centuries as the most effective measure for the treatment, yet the infection rate is still high. Furthermore, synthetic drugs may cause several disadvantages, including drug resistance, adverse drug reaction, chemical residue, and high cost (Panyarachun et al., 2010). All of these problems could be resolved by using common traditional herbs to replace synthetic drugs which would be more acceptable, easier to obtain, and low-cost.
Nigella sativa Linn. has been used as a traditional medicine in the Middle East and South Asia for over 100 years, and it has been introduced and utilized in other parts of the world as one of the most promising treatment as antihypertensive, diuretics, analgesic, antibacterial, anti-diarrheal, immunostimulatory, bronchodilator, liver and gastro protectant, and anthelmintic properties (Boskabady et al., 2010; Abdel-Zaher et al., 2011; Ahmed et al., 2013; Ullah et al., 2017).
The present study aimed to investigate the efficacy of N. sativa seed extract as anthelmintic against a naturally acquired Paramphistomum cervi infection in cattle in Indonesia.
MATERIALS AND METHODS
Collection of Parasites
The flukes of P. cervi were collected from the rumen of Aceh cattle from the local municipality abattoir. The flukes were stored in PBS solution and transported to the parasitology laboratory in a protected container. The flukes were rinsed three times with 0.85% NaCl (Anuracpreeda et al., 2008), and examined immediately to avoid any disruption.
Plants Extraction
Nigella sativa Linn. seed was purchased from local market in Banda Aceh, imported from India. Five hundred grams of dried seed of N. sativa were extracted using 96% methanol as explained by Balqis et al. (2017), and Hussain et al. (2011). The plants were soaked in 2 L of methanol for three days at room temperature. The filtrate was then collected and the solvent was removed by rotary evaporator at 40 oC. The crude extract was re-suspended in phosphate buffer saline (PBS) and diluted to several concentrations (5%, 10%, 25%, and 40%).
Anthelmintic assay on Paramphistomum cervi
Four different concentrations of N. sativa seed extract were used T1 (5%), T2 (10%), T3 (25%), and T4 (40%). Albendazole (Valbazen, Shandong Huahai Biotechnology Co. Ltd., Republic of China) 0.24 mg/ml was used as positive control (C1), and PBS was used as negative control (C2). The flukes were placed in petri dishes for each treatment and incubated at room temperature. The observation was conducted every 15 minutes until all flukes died. The fluke’s motility was recorded by score index, and the mortality time was recorded. The motility was scored according to Kuichi et al. (1987) and Jiraungkoorskul et al. (2005). Score 3 is given when the whole body is moving, score 2 is granted when only parts of body moving, score 1 if the fluke is moving when induced with warm water (60 oC), and score 0 when the fluke is completely dead.
Specimen Preparation for Histopathology Examination
The flukes were further examined for histopathological changes after N. sativa seed extract treatment. The flukes were set for paraffin embedding and fixed in 10% formalin for 24 hours, then dehydrated in ascending concentration of ethanol. The samples were cleared by xylol, and then embedded in paraffin wax. The samples were dissected longitudinally at 3-5 µm thickness and stained with hematoxylin and eosin. The observation of abnormalities was carried out on each section under a light microscope (Olympus, Tokyo, Japan), and photographed (Hayashi et al., 2017).
Statistical Analysis
Results were expressed as mean ± standard deviation (SD) and were evaluated for significance using a one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test (P ≥ 0.05).
RESULTS
Motility Observation
Paramphistomum flukes motility is relatively easy to observe in vitro. In treatment groups, the flukes movement was more gradual than negative control group. Methanolic extract of N. sativa 25% (T3) and 40% (T4) caused decreased motility of Paramphistomum cervi after 20 minutes of treatment, and all flukes died within 80 minutes. For 5% (T1) and 10% (T2) extract, the motility was decreased after 30 minutes of treatment, within 90 minutes, all flukes had died. In positive control (C1), the motility was declined after 60 minutes of treatment, and after 110 minutes, all flukes died. This group had longer time to succumb than treatment group. In negative control, the flukes were stable even after 110 minutes, there was no sign of reduced motility. The movement started to decline after 200 minutes of treatment, and after 8 hours, the flukes finally died. ANOVA test showed significant difference (p<0.05) between negative control and treatment groups. When compared within groups, positive control and T1, T2, T3, T4 were significantly different (p<0.05). The graphic of mean fluke motility and mortality from each group is provided in Figure 1.
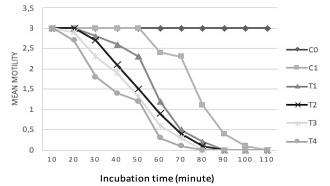
Figure 1: Mean motility dan mortality time of Paramphistomum cervi. C0= negative control (PBS); C1= positive control (albendazole); T1= 5% of Nigella sativa extract; T2= 10% of Nigella sativa extract; T3= 25% of Nigella sativa extract; T4= 40% of Nigella sativa extract.
Histopathology studies
From histopathological observation, there are some alterations occurred within flukes’ organs. The teguments of flukes in 25% and 40% extract showed lysis of tegument and absence of papillae. There were also disruptions of parenchyma cells indicated by disappearance of cells, and swelling of the tegument. In 5% and 10% of treatment, the teguments were still intact, however, moderate swelling was observed. In 5% extract, no damaged cells were noticed, while in 10% extract, some damaged parenchyma cells were spotted. In positive control, disintegrated tegument was clearly seen, while in negative control, tegument was intact with healthy papillae and no sign of swelling (Figure 2)
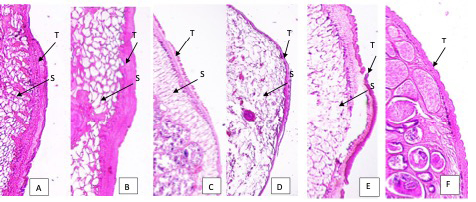
Figure 2: Tegument of Paramphistomum cervi after treatment. A: 5% extract; B: 10% extract; C: 25% extract; D: 40% extract of Nigella sativa. E. Positive control (albendazole) F. Negative control (PBS). T: tegument; S: swelling of tegument
Reproductive organs were also affected by the extract. Testes of flukes were disintegrated in 25% and 40% extract; spermatozoa cells showed erosion. In negative control, the testis was still intact, filled with spermatozoa cells, while in positive control, erosion of cells was observed (Figure 3).
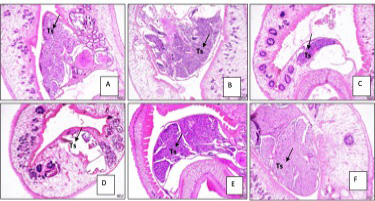
Figure 3: Reproductive organs of Paramphistomum cervi after treatment. A. 5% extract B. 10% extract. C. 25% extract. D. 40% extract. E. Negative control. F. Positive control. Ts: testes.
Regarding the sucker, some changes were slightly different among treatment groups, while it was clearly different between negative control and treatment groups. In negative control group, the sucker had a thick muscular rim covered with folds and papillae. In T4, the muscular rim was severely damaged, and anterior tegument has lost almost all the papillae (Figure 4).
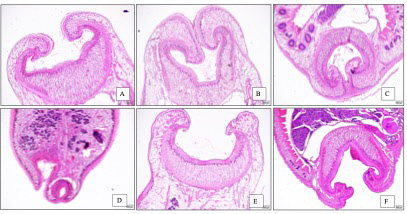
Figure 4: Cell lysis and lipid degeneration of Paramphistomum cervi after treatment. A. 5% extract B. 10% extract. C. 25% extract. D. 40% extract. E. Negative control. CL: cell lysis. LD: lipid degeneration.
Discussion
Chemical composition of N. sativa seed consists of quinones, monoterpene hydrocarbons, sesquiterpene hydrocarbons, diterpenes, fatty acids, thymoquinone, thymohydroquinone, thymol, and dithymoquinone (Venkatachallam et al., 2010). The composition of N. sativa extract, especially thymoquinone was reported to affect the metabolisme of trematode, especially tegument (Mostafa and Soliman, 2010; Ullah et al., 2017).
Tegument is part of trematode fluke that is directly linked to host tissue and body fluid. Tegument plays role in homeostasis such as nutrient intake and the regulation of osmosis. It also has function in protection against host digestive enzymes and immune responses (Panyarachun et al., 2010). Understanding the changes in tegument structure due to anthelmintics activities is essential in developing any potential drugs or vaccine which may obstruct the trematode’s tegument. In this study, N. sativa extract showed convincingly potent activity in damaging tegument of Paramphistomum cervi. N. sativa 40% showed severe effects towards tegument, indicated by disappearance of papillae on the surface of tegument. This result confirmed that N. sativa extract at this concentration had potential flukicidal activity because it affected the tegument directly. Besides tegument, N. sativa extract also damaged reproductive organs of P. cervi, indicated that this extract had penetrated inside the parencyme and disrupt internal organs as well. We also suggest the use of combination of N. sativa extract with available synthetic drugs to obtain higher efficacy. Shalaby et al. (2012) reported that a combination of N. sativa oil and ivermectine had severe in vitro effect on F. gigantica, especially on the tegument part of flukes.
Numerous studies have been reported about the effective use of N. sativa extract to treat helminth infection in South Asia and Middle-East. Shalaby and El-Moghazy (2013) reported the use of N. sativa oil (NSO) to treat Toxocara vitulorum infection. They found swelling and severe disorganization of cuticle and body musculature, which exhibited nematocidal effect of NSO. Another report by Ullah et al. (2017) exhibited that thymoquinone from N. sativa extract had the ability to reduce F. gigantica motility and caused disruption of tegument as well as spine erosion in the posterior region and around acetabulum. A study by Hossain et al. (2012), stated that the extract of Dregea volubilis had flukicidal effect on P. explanatum by severe distortion on tegument surface. These reports are similar to our results, where N. sativa extract also caused tegument disruption. Tegument is a substantial organ for trematodes especially in respiration and metabolic function. Tegument also has a role in suppressing host immune system, and driving inflammatory response associated with immunopathology (Hamilton et al., 2009).
Shalaby et al. (2010) reported severe damage of oral sucker and acetabulum of P. microbothrium so that little recognizable part remained after treatment with artemeter. In our study, the changes of oral sucker and acetabulum due to N. sativa extract were relatively mild, as only small part of anterior and posterior of the flukes affected histopathologically. Winkelhagen et al. (2012) reported that flukes resistance from human infections had occurred, therefore it was important to find other sources of anti-parasitic drugs, especially from natural products to avoid drug resistance. He further stated that there is long association between parasite infection, host, and herbal remedies.
CONCLUSION
From the results it was shown that extract of N. sativa had the ability to suppress flukes’ motility, the influence was even better than positive control.The extract showed good anthelmintic property, flukes’ motility was decreased gradually, and all flukes died within 80 minutes. Histopathology examination showed some alterations of flukes’ organs, especially on tegument, where it caused tegument disruption and papillae disappearance All of these alterations caused severe damages on flukes and lead to death. The concentration recommended for paramphistomiasis is 25% of N. sativa extract, which is considered as proper concentration that will not interfere with rumen microorganism.
ACKNOWLEDGEMENT
Author wishes to thank all the technical staff in the Laboratory of Pharmacology, Parasitology, and Laboratory of Research, Faculty of Veterinary Medicine, USK, for their assistance. Author is thankful to the Faculty of Veterinary Medicine, USK, for supporting this research. We declare that this article has not been published in other journal.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
MH contributed in project design and manuscript preparation. HV contributed in data analysis and finalizing of manuscript. SRA contributed in field coordination, laboratory works, and data entry. All authors agree to the conditions outlined in the copyright assignment form.
ETHICAL APPROVAL
All procedures performed in this study were in accordance with the ethical standards of the Faculty of Veterinary Medicine, Universitas Syiah Kuala. No specific permissions were required. Samples were collected in slaughter houses in Banda Aceh following standard procedures.
REFERENCES






