Advances in Animal and Veterinary Sciences
Research Article
Clinical, Haemato-biochemical, and Histopathological Studies on some Dermopathies in Dogs
Mohamed Ahmed A.Fouda1, Hitham Abdel-Saeed2*, Sherein S. Abdelgayed3, Ossama Mohamed Abdou2
1MD student at Department of Internal Medicine, Faculty of Veterinary Medicine, Cairo University; 2Department of Internal Medicine, Faculty of Veterinary Medicine, Cairo University, Egypt; 3Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Egypt.
Abstract | Almost all dermatology patients have a primary or underlying disease that causes secondary infections. In this study, primary dermatological diseases were investigated as well as concurrent secondary infections to reach a definitive diagnosis that will lead to proper treatment and cure. The present work included 65 dogs which divided into 2 groups. Control group (20 apparently healthy dogs) and diseased group (45 dogs). All dogs were clinically examined; blood samples were collected for estimation of CBC (Complete blood count), ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), GGT (gamma-glutamyl transferase), BUN (Blood urea nitrogen), Creatinine, Thyroid profile FT4 (free thyroxine-4), TSH (thyroid stimulating hormone) and Cortisol. Only diseased dogs were subjected to skin scraping examination while biopsies from skin lesions were obtained for histopathological examination. Results showed significant (P ≤0.01) leukocytosis in diseased group compared to control one. Skin scraping revealed mites infestation (sarcoptic mite), (Demodectic mite: demodex canis). Also, ectothrix and endothrix spores of dermatophytes were detected (Microsporum canis). Histopathological examination revealed various changes in epidermal as well as dermal layer in different dermopathies. Conclusion: the present work indicated that, clinical examination, skin scraping and laboratory tests (CBC, Biochemistry and hormonal profiles) for different dermopathies in dogs will drive to initial diagnosis, whereas histopathological examination for skin biopsies will guide to a definite diagnosis and furthermore specific therapeutic strategies.
Keywords | Dermopathies, Skin biopsy, Haemato-biochemical, Histopathology, Dogs.
Received | September 05, 2020; Accepted | September 16, 2020; Published | December 10, 2020
*Correspondence | Hitham Abdel-Saeed, Department of Internal Medicine, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: hythamabdu5@gmail.com
Citation | A Fouda MA, Abdel-Saeed H, Abdelgayed SS, Abdou OM (2021). Clinical, haemato-biochemical, and histopathological studies on some dermopathies in dogs. Adv. Anim. Vet. Sci. 9(1): 94-102.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.94.102
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abdel-Saeed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Skin is the most sensitive part of dog body and has tremendous aesthetic value. In small animal practice, a majority of ailments are constituted by dermatological abnormalities as the concurrent or chief owner’s complaint (Sakina and Mandial, 2011).
Skin disorder is a one of the most commonly recorded problems in pet animals. Dogs are susceptible to various skin problems which may be parasitic, fungal and bacterial as well as allergies of various origin. These conditions become worse in hot and humid climate making them hard to be resolved. This not only deteriorates the cosmetic appearance of the animal but also possess a risk to its general health and utility (Thapa and Sarkar, 2018).
Skin microbiome may be the source of secondary infections that can influence the severity of canine inflammatory and pruritic allergic skin disorders. Microbial culture-based studies showed that the most prominent bacteria on skin lesions of dogs with atopic dermatitis is Staphylococcus pseudointermedius, whereas Malassezia pachydermatitis is the main fungal representative (Miller et al., 2012). Topical antimicrobial therapy is widely used to relieve symptoms of atopic dermatitis partly caused by secondary infections (Chermprapai et al., 2019).
Diagnosis of skin disorders is usually the furthermost challenging part of dermatology. Diagnostic criteria depends on both clinical and microscopic features of skin disorders (Umarji et al., 2018).
Cutaneous biopsy evaluation can be an advantage for the practitioner with the aid of experienced pathologist. The practitioner can improve the diagnostic reliability of skin biopsies by properly selecting lesions for biopsy and providing the pathologist with a complete clinical differential diagnosis list. Cutaneous biopsy has the potential to provide the greatest amount of information in the shortest period of time. (Hnilica and Patterson, 2016).
Aim of Work
The present study was performed to evaluate the diagnostic criteria of histopathological changes among different dermopathies in dogs and find their correlations with clinical examination, Skin scraping, haemato-biochemical criteria as well as hormonal profile.
Materials and methods
Animals
This study was performed on 65 dogs of different breeds and sexes (39 males and 26 females) with ages ranged from 6 months to 5 years, dogs were admitted to small animal clinic, Faculty of veterinary medicine, Cairo university, Egypt and small animal private veterinary clinics in Egypt from August 2018 to March 2020.The animals were divided into two groups. First group (control group) consisted of 20 apparently healthy dogs and 2nd group (diseased group) consisted of 45 dogs with different dermopathies.
Clinical Examination
In the present study case history, general clinical examination were applied for all body parts and systems of all animals. Special clinical examination and scrutinization of skin was performed as well.
Skin Scraping Samples
Both superficial and deep skin scrapings from each diseased dog were examined using 4X, 10X and 100X power lens microscope according to Hnilica and Patterson (2016).
Skin Swab Samples
Clean and sterile skin swabs were taken from some diseased cases. Also, ear swabs were obtained in some cases with ear lesions for isolation and identification of causative agents.
Skin Biopsy
Skin biopsies samples were collected from some affected skin lesions of diseased dogs using Punch biopsy (4 mm to 6 mm diameter) for histopathological examination after sedation using xylazine 1mg/kg intramuscular injection and lidocaine 2% 1ml subcutaneous injection around the lesion from which the tissue biopsy will be obtained according to clinical handbook of canine dermatology (Jasmin, 2011).
Blood and Serum Samples
Blood samples were obtained from both healthy and diseased dogs. First part of blood was collected on EDTA blood tube for examination of erythron, leukon and thrombon using veterinary hematology analyzer. Second part was collected in plain blood tube for serum separation and estimation of ALT, AST and GGT, BUN, Creatinine, Thyroid profile FT4 (free thyroxine-4) TSH (thyroid stimulating hormone) and Cortisol level according to specific test kits (spectrum diagnostics, Egypt).
Histopathological Examination
Skin biopsies from diseased dogs were fixed in neutral buffered formalin 10%, dehydrated in ascending grade of alcohol, cleared and embedded in paraffin, sectioned at 5 mm thickness and stained by Hematoxylin and Eosin and examined microscopically according to Bancroft et al. (1996).
Statistical Analysis
All the data was added to Microsoft Excel sheet and results were shown as mean± SE (Standard Error). Diseased animals were compared with control animal’s data using student T- Test through SPSS program version 16. P value ≤ 0.001, 0.01 and 0.05 were considered significant.
Ethics Statement
The use of dogs in this study was permitted by the owners of the dogs. All animal procedures were performed in accordance with the Guidelines of department of medicine, Faculty of veterinary medicine, Cairo University, Egypt.
Results
The present study showed the different dermatopathies in diseased group (Table 1). Study included 45 dogs (27 males and 18 females) from different breeds (18 Mixed breed,6 German shepherd, 5 Rottweiler, 5 Pitbull, 2 Griffon, 1 Beagle, 1 Alapay, 1 Doberman pincher, 2 Golden retriever, 1 Great Dane, 1 German shepherd mixed husky,
Table 1: Incidence of dermatopathies in diseased dogs
| Lesion | Number | % |
| Hot spot (superficial pyoderma) | 7 | 15.5% |
| Dermatophytosis (ringworm) | 6 | 13.3% |
| Dermatitis due to flea infestation (FAD) flea allergic dermatitis | 5 | 11.1% |
| Heavy ticks infestation with dermatitis a | 4 | 8.9% |
| Dermatophytosis with surface pyoderma | 3 | 6.7% |
| Chronic otitis externa | 3 | 6.7% |
| Canine cutaneous papillomatosis | 2 | 4.4% |
| Sarcoptic mites | 2 | 4.4% |
|
Juvenile onset generalized pyodemodicosis |
2 | 4.4% |
| Bacterial pododermatitis | 1 | 2.2% |
| Contact dermatitis | 1 | 2.2% |
| Deep pyoderma | 1 | 2.2% |
| Food allergy+Otitis externa | 1 | 2.2% |
|
Hair loss due to anaemia |
1 | 2.2% |
| Malasezia pachydermatitis+Fibroma | 1 | 2.2% |
| Sarcoma | 1 | 2.2% |
| Mammary solid adenocarcinoma | 1 | 2.2% |
| Traumatic dermatitis with secondary bacterial infection | 1 | 2.2% |
| Acanthiosis negricans | 1 | 2.2% |
| Demodicosis+ringworm | 1 | 2.2% |
| Total | 45 |
100% |
1 Labrador retriever and 1 Saint Bernard). There was a significant increase in platelet count in diseased group compared to control one (P≤0.001). Significant leukocytosis was found in diseased group (P ≤0.01) which revealed a significant increase in Lymphocytes (P≤0.01), Eosinophils (P≤0.001) and significant increase in number of Basophils (P≤0.01) compared to control group (Table 2). Also there was a significant increases in ALT (P≤0.05) in diseased group compared to control one (Table 3). On the other hand, non-significant changes were found in hormonal profiles (FT4, TSH and cortisol) (Table 4).
Regarding skin scraping samples, results revealed presence of Sarcoptes scabiei and Demodex canis adult mites as well as dermatophytes infection included the presence of ectothrix and endothrix spores that were appeared via microscopic examination as shown in Fig.7. In the term of clinico-histopathological findings, clinical examination revealed many pathological conditions which confirmed by histological findings after microscopic examination. Bacterial infection dermopathies were commonly diagnosed
Table 2: Hematological parameters of diseased dogs compared with apparently healthy (control) dogs
|
Animals Parameters |
Control dogs | Diseased dogs | |
|
RBCs (×1012/L) |
5.67 ±0.71 |
4.85 ±1.14 |
|
| HB (g/L) |
146.80 ±18.50 |
113.40 ±25.00 |
|
|
Thrombocytes(×109/L) |
251.71 ±75.20 |
271 ±136.65*** |
|
| MCV |
70.98 ±6.31 |
69.59 ±6.72 |
|
| MCH |
26.03 ±3.05 |
22.90 ±1.94 |
|
| MCHC |
36.66 ±2.59 |
32.94 ±2.44 |
|
|
WBCs (×109/L) |
11.16 ±2.00 |
14.25 ±5.63** |
|
|
(DLC) Differential leuckocytic count |
Lymphocytes (%) |
25.21 ±7.25 |
32.69 ±18.80** |
| Staff |
3.95 ±2.27 |
4.41 ±2.92 |
|
| Segmented |
62.79 ±7.94 |
52.91 ±15.45 |
|
| Monocytes (%) |
5.63 ±2.91 |
7.51 ±4.87 |
|
| Eosinophils (%) |
2.42 ±0.96 |
3.82 ±2.04*** |
|
| Basophils (%) |
0.00 ±0.00 |
0.12 ±0.54** |
|
***P≤0.001 **P≤0.01 *P≤0.05
Table 3: Biochemical parameters of diseased dogs compared with apparently healthy (control) dogs
|
Animals Parameters |
Control dogs |
Diseased dogs
|
|
ALT (µkat/L) |
0.09±0.28 | 0.99±0.53* |
|
AST (µkat/L) |
0.17±0.28 | 0.62±0.25 |
|
GGT (µkat/L) |
0.05±0.28 | 0.07±0.03 |
|
Creatinine µmol/L |
15.25±1296.25 |
76.25+ or - 0 |
|
BUN µmol/L |
417.85±1296.25 | 1677.50±305 |
***P≤0.001 **P≤0.01 *P≤0.05
Table 4: Hormonal parameters of diseased dogs compared with apparently healthy (control) dogs
|
Animals Parameters |
Control dogs |
Diseased dogs
|
|
Free T4 (pmol/L) |
16.47±6.44 | 12.48±7.21 |
|
TSH (mIU/L) |
0.43±0.29 | 0.70±0.70 |
|
Cortisol (nmol/L) |
42.21±31.17 | 51.31±35.86 |
***P≤0.001 **P≤0.01 *P≤0.05
(Fig. 2) and clinically represented by dermatitis with secondary superficial pyoderma which grossly showed two pruritic circumscribed alopecic lesions with erythema and hair loss in the front of the head (Fig. 2a). Histologically the lesion was manifested by Eosinophilic dermatitis with infiltration of eosinophils in the dermal layer, keratolysis and spongiosis with swelling of epidermal cells with clear cytoplasm [Fig. 2(a1) & 2(a2)]. A clinical case of a dog suffering from traumatic dermatitis with secondary bacterial infection was recorded (Fig. 1b) histological lesions revealed Lymphocytic dermatitis with infiltration of lymphocytes in the dermal layer [Fig. 2(b1)] together with Keratolysis, acantholysis, lysis of keratin and spongiosa cell layer reflected in severely atrophied epidermis [Fig. 2(b2)]. A dog with superficial pyoderma and ulceration (Fig. 2C) denoted lymphocytic dermatitis with infiltration of lymphocytes in the dermal layer [Fig. 2(c1) & 2(c2)]. Erythematous lesions with hair loss alopecic areas were another reported clinical cases (Fig. 3), some of them reported in the right elbow (Fig 3a) and showed acanthosis with increased thickness of spongiosa cell layer and spongiosis noted by the swelling in epidermal cells with clear cytoplasm [Fig 3(a1)]. Perivascular lymphocytic dermatitis with accumulation of lymphocytes around the congested blood vessel in the dermal layer were denoted [Fig. 3(a2)]. Another oval area of non-pruritic hair loss was detected in the back (Fig. 3b), of which skin biopsy showed ulceration with deep destruction in the epidermal layer together with dermal necrosis [Fig. 3(b1)], keratolysis and acantholysis with atrophied epidermis were also reported [Fig. 3(b2)].
Figure 1: Mites and dermatophytes showing; 10 (a), (a1) and (a2) showed Demodex, (b) showed Microsporum and (b1), (b2) showed Sarcoptic mites.
Parasitic infestations were recorded in many other clinical cases (Fig. 4 & 5). Three cases of flea allergic dermatitis (FAD) wisth pruritic lesion on the left trunk was reported (Fig. 4a &4b & 5a). Microscopic examination of skin biopsies showed lymphocytic dermatitis with accumulation of lymphocytes in the dermal layer [Fig. 4(a1)], ulceration together with dermal necrosis were also reported [Fig. 4(a2) & 4(b1)], necrotic hair follicle [Fig. 4(b2)], keratolysis with complete destruction in keratin layer and spongiosis
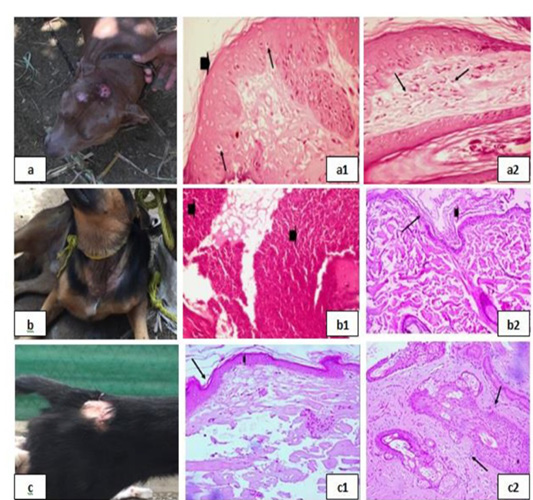
Figure 2: Bacterial infection dermopathies showing; 2a: Erythema and hair loss in the front of the head. 2(a1): keratolysis (arrow head) and spongiosis (arrows), (H&E X 200). 2(a2): Eosinophilic dermatitis with accumulation of eosinophils in the dermal layer (arrows), (H&E X 200). 2b: Traumatic dermatitis with secondary bacterial infection. 2(b1): Lymphocytic dermatitis (arrows), (H&E X 200). 2(b2): Keratolysis (arrow head) and acantholysis (arrow), (H&E X 200). 2c: Superficial pyoderma and ulcer formation. 2(c1), (c2): Lymphocytic dermatitis (arrows), (H&E X 200).
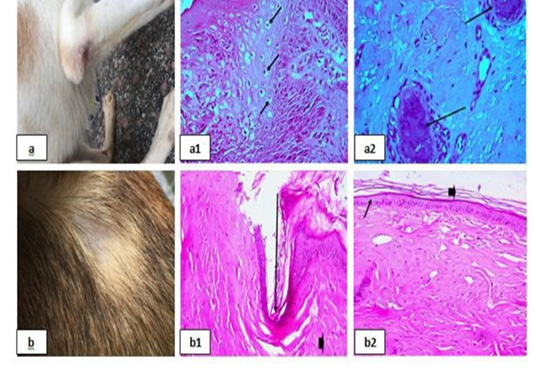
Figure 3: Erythematous lesions with hair loss alopecic areas showing; 3a: Erythematous lesions in the right elbow. 3(a1): Acanthosis and spongios (arrows), (H&E X 200). 3(a2): Perivascular Lymphocytic dermatitis (arrows), (H&E X 200). 3b: Erythematous lesions in the back. 3(b1): Ulceration (arrows), (H&E X 200). 3(b2): keratolysis (arrow head) and acantholysis (arrows), (H&E X 200).
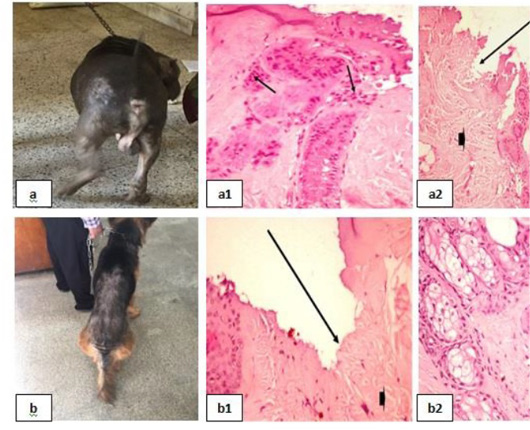
Figure 4: Parasitic infestations 1 showing; 4a, 4b: Flea allergic dermatitis (FAD) with pruritic lesion on the left trunk. 4(a1): Lymphocytic dermatitis (arrows), (H&E X 200). 4(a2), (b1): Ulceration (arrows), (H&E X 200). 4(b2): Necrotic hair follicle, (H&E X 200).
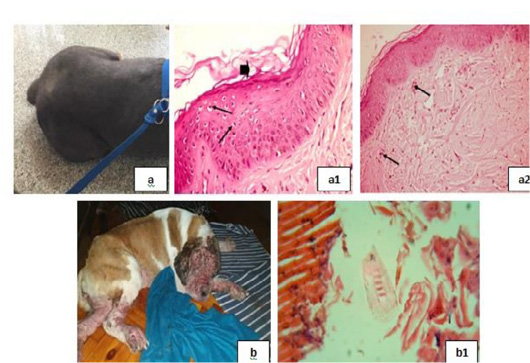
Figure 5: Parasitic infestations 2 showing; 5a: Flea allergic dermatitis (FAD) with pruritic lesion on the left trunk. 5(a1): Keratolysis (arrow head) and spongiosis (arrows), (H&E X 200). 5(a2): Eosinophilic dermatitis (arrows), (H&E X 200). 5b: Pyodemodicosis 5(b1): Demodex, (H&E X 200).
with swelling in epidermal cells and clear cytoplasm [Fig. 5(a1)], and eosinophilic dermatitis with accumulation of eosinophils in the dermal layer [Fig. 5(a2)] were recorded. A case of pyodemodicosis was recorded [Fig. 5b] and demodex was appeared by microscopic examination of biopsy [Fig. 5(b1)].
Dermatophytosis was also detected in many clinical cases (Fig. 8). Dermatophytosis with surface pyoderma and non-pruritic alopecia in the dorsal base of the neck was recorded (Fig. 8a), skin biopsy showed lymphocytic dermatitis with infiltration of lymphocytes in the dermal layer together with increased number of adipose cells [Fig. 8 (a1)]. Dermatophytosis with surface pyoderma and circumscribed non pruritic white patches was distributed on frontal region, dorsal plantar, ear pinna, and left thigh (Fig. 8b), skin biopsy showed necrotic dermatitis with sever necrosis in the dermal layer and leucocytic cells infiltrations [Fig. 8 (b1)].
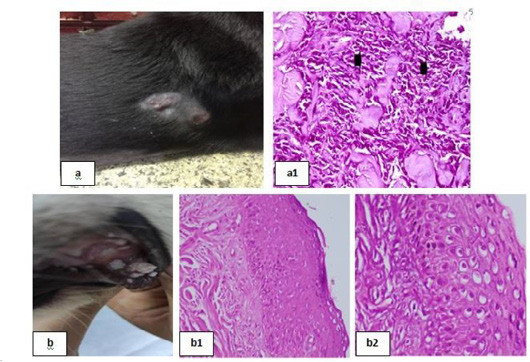
Figure 6: Skin tumors 1 showing; 6a: Soft subcutaneous lumps. 6(a1): Sarcoma cell tumor in the dermal layer with sheets of malignant cells, note the pleomorphism, hyperchromacia, increase nucleous : cytoplasmic ratio (arrow head), (H&E X 200). 6b: Cutaneous papillomatosis in the mouth 6(b1): Hyperplastic epidermis supported by fibrovascular tissue in the dermis, (H&E X 200). 6(b2): Irregular intracytoplasmic keratohyaline granules, (H&E X 200).
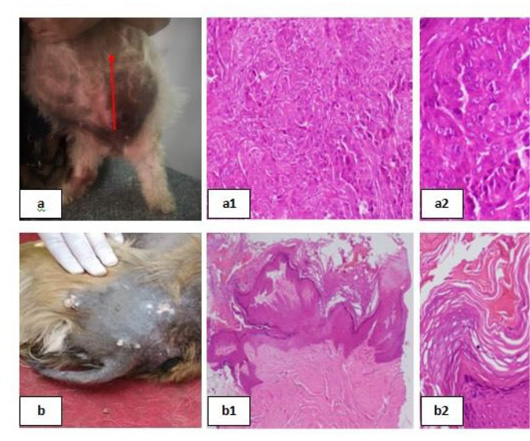
Figure 7: Skin tumors 2 showing; 7a: Mammary solid adenocarcinoma 7(a1): Carcinoma, solid type, (H&E X 200). 7(a2): Pleomorphic cells, hyperchromatic nuclei, central prominent nucleolus, and frequently detected mitosis, (H&E X 200). 7b: Canine papilloma, 7(b1): Hyperplastic epidermis , (H&E X 200). 7(b2): Prominent hyperkeratosis , (H&E X 200).
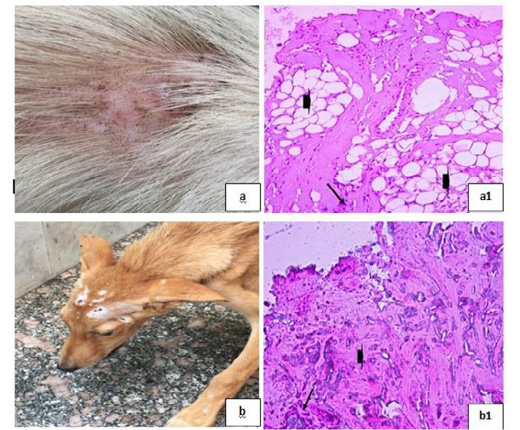
Figure 8: Dermatophytosis showing; 8a: Dermatophytosis with surface pyoderma and non-pruritic alopecia 8(a1): Lymphocytic dermatitis (arrow), with increased number of adipose cells (arrow head), (H&E X 200). 8b: Dermatophytosis with surface pyoderma and circumscribed white patches 8(b1): Necrotic dermatitis with sever necrosis in the dermal layer (arrow head) and leucocytic cells infiltrations (arrow), (H&E X 200).
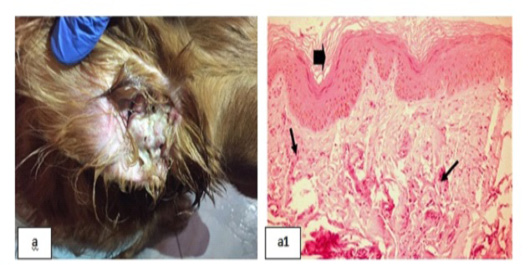
Figure 9: Chronic Otitis externa showing; 9a: Red inflamed ear. 9(a1): Dyskeratosis (arrow head) and eosinophilic dermatitis (arrows), (H&E X 200).
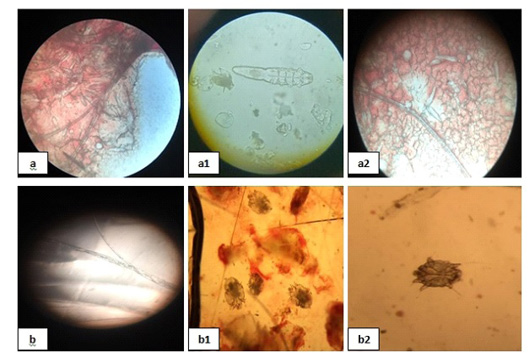
Figure 10: Mites and dermatophytes showing; 10 (a), (a1) and (a2) showed Demodex, (b) showed Microsporum and (b1), (b2) showed Sarcoptic mites.
Chronic Otitis externa was detected (Fig. 9a), skin biopsy showed dyskeratosis with disorganized keratin layer and eosinophilic dermatitis denoted by the infiltration of eosinophils in the dermal layer [Fig. 9(a1)].
Mites and dermatophytes were detected in many clinical cases (Fig. 10) including Demodex canis, Microsporum canis, and Sarcoptic scabiei adult mites [Fig. 10 (a), (a1), (a2), (b), (b1), and (b2)].
Skin tumors were detected in many clinical cases (Fig. 6 & 7). Haemangiosarcoma with soft subcutaneous lumps all over the body which were centrally depressed (Fig. 6a) was reported, microscopic examination of skin biopsies revealed sarcoma cell tumor in the dermal layer with sheets of malignant cells noted by pleomorphism, hyperchromacia and increase nucleus cytoplasmic ratio [Fig. 6(a1)]. Cutaneous papillomatosis in the mouth was detected (Fig. 6b), microscopically, there were hyperplastic epidermis supported by fibrovascular tissue in the dermis [Fig. 6(b1)] and irregular intracytoplasmic keratohyaline granules [Fig. 6(b2)]. Mammary solid adenocarcinoma with solid mass in the mammary gland and ulceration of the skin was detected (Fig. 7a), microscopic examination revealed carcinoma, solid type [Fig. 7(a1)] and pleomorphic cells showed hyperchromatic nuclei, central prominent nucleolus, and frequently detected mitosis [Fig. 7(a2)]. Canine papilloma, Nodular masses on the lateral side of the thigh with alopecia in groin and ventral abdomen with black discoloration were recorded (Fig. 7b), skin biopsy showed hyperplastic epidermis projects ventrally into the dermis [Fig. 7(b1)] together with prominent hyperkeratosis [Fig. 7(b2)].
Discussion
The skin is the largest and most visible organ of the body, it is the anatomic and physiologic barrier between animal and the surrounding environment, it provides protection from physical, chemical and microbiologic injury and it is sensory component perceive heat, cold, touch, pressure and pruritus.in addition, the skin is synergistic with internal organ system and thus reflect pathologic processes that are either primary or shared with other organs of the body (Miller et al., 2012). The present study revealed statistically significant increase in platelet count (in diseased group 45 dogs compared to control one) but in comparison with the reference value it was not of clinical significance. Also, there were significant leukocytosis with significant increase in the numbers of eosinophils, lymphocytes and basophils. These findings were similar to Sharma and Pokharel (2019) who stated that the haematogical parameters revealed leukocytosis might be due to inflammation and secondary bacterial infections. Leukocytosis and similar findings were also reported by Arora et al. (2013); Nikee et al. (2018).
The present results recorded a significant increase in ALT, this was due to the administration of short term corticosteroids by the dog owners as a treatment for skin allergy as stated by Mochel (2017). Such an increase was evident in serum ALT activity at day 14 only (P ≤ 0.01) after administration of short term corticosteroids (Mochel, 2017).
Although there were no significant changes between the two groups in hormonal parameters but hypothyroidism, hyperadrenocorticism is responsible for many canine dermatologics. Canine endocrine dermatologics represent 8.6% of the dermatological diseases, most commonly presenting classic symptoms of non-pruritic bilaterally symmetrical alopecia which develops chronically. The most occurring are hypothyroidism, hyperadrenocorticism (HAC) and hyperestrogenism. (Costa et al., 2016). Skin scraping showed the presence of Demodex canis (Salem et al., 2020), Sarcoptic scabiei mite, otodectic mites and dermatophytes in different dermopathies as stated by (Barillas et al., 2019)
Most cases revealed eosinophilic dermatitis and / or lymphocytic infiltration with destruction in keratin layer or destruction of the basement membrane. Other samples showed acanthosis or hyperkeratosis and spongiosis that similar to the results of Bond et al. (2020). Dogs with hot spot and surface pyoderma showed eosinophilic dermatitis as well as acute superficial lesions with necrosis or ulceration of the epithelium and edema of the upper dermis with eosinophilic dermatitis which come in coincidence with Holm et al. (2004) and Olivry and Hill (2001).
High numbers of eosinophils were common in type I hypersensitivity, this picture appeared in allergic dermopathies consisted of pruritic cutaneous diseases resulting from immunological reactions to different types of antigens. Allergopathies comprising ectoparasites allergy (EA), canine atopic dermatitis (CAD), food allergy (FA) and contact dermatitis (CD) that recorded by Ferreira (2017); Tizard (2014).
Spongiosis was a characteristic histopathologic appearance in eczematous dermatitis which come in agreement with the results of Trautmann et al. (2001); Astner et al. (2005). The histopathological lesions associated with canine allergic dermatitis involve epidermal and dermal alterations. In epidermis, the most common findings involve parakeratotic and orthokeratotic hyperkeratosis, acanthosis, spongiosis and hypermelanosis. In dermis, there were lymphocytes and monocytes. All these findings were in the line with Ferreira (2017).
Haemangio-sarcoma is a malignant vascular tumor that arises from endothelial cells of any organ with a blood supply. The most common primary sites included spleen, right atrium of the heart, sub-cutis and dermis. The occurrence of cutaneous haemangiosarcoma is least common among visceral tumors. Haemangio-sarcoma represents 2 to 3% of all cutaneous tumors in dogs which recorded by Prasath et al. (2018). Sarcoma cell tumor in the dermal layer with sheets of malignant cells noted by pleomorphism, hyperchromacia, increase nucleus-cytoplasmic ratio which indicated the presence of malignant cell in the dermis and sub-cutis as stated by Prasath et al. (2018). Dogs developed a variety of cutaneous neoplasms; the etiology of many was unknown. Papillomavirus antigens have been detected in cutaneous squamous papilloma and some squamous cell carcinoma as described by Campbell (1988). There were two cases with skin masses have the histopathological picture of papilloma with hyperplastic epidermis supported by fibrovascular tissue in the dermis, cells with irregular intracytoplasmic keratohyaline granules as stated by Sharun et al. (2020) who reported that canine oral papillomatosis is generally diagnosed based on the histopathological findings. One case has a mass in the mammary gland showed carcinoma of solid type. The cells have hyperchromatic nuclei and with central prominent nucleolus, showed frequently detected mitosis which come in the same context of Mohapatra et al. (2016).
Conclusion
As a corollary, it could be concluded that thorough clinical examination which was done routinely in small animal clinic is of paramount importance, but it can in most cases give only tentative diagnosis which is of a little value in treatment’s formulation. A step forward is quick laboratory tests which afford a better step for diagnosis. All these preceding steps may not be satisfactory for definite diagnosis. The final step without hesitation or argument is histopathology where upon lesions on cellular and interstitial levels could be scrutinized, assed and determined.
Acknowledgment
The authors express their gratefulness to department of Internal Medicine, faculty of Veterinary Medicine, Cairo University, Egypt. Great thanks to Dr. Hitham Abdel-Saeed and Prof. Dr. Sherein S. Abdelgayed for their guidance and follow up in all procedures.
Conflict of interest
The authors declare that they have no conflict of interest.
authors contribution
Mohamed Ahmed A. Fouda carried out the practical work of the study and writing the manuscript. Hitham Abdel-Saeed share in preparing the manuscript. Sherein S. Abdelgayed carried out the histopathological examination. Ossama Mohamed Abdou Supervise and guiding the manuscript writing and the whole work
REFERENCES






