Advances in Animal and Veterinary Sciences
Research Article
Subacute Ruminal Acidosis in Feedlot: Incidence, Clinical Alterations and its Sequelae
Noura El- Shahat Abd El- Rahman Attia
Animal Medicine Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig - 44511, Egypt.
Abstract | This study was aimed to determine the incidence of subacute rumen acidosis (SARA) in beef cattle and its sequelae in the herd. Subacute ruminal acidosis (SARA) has become an economically important and common problem in well-managed bullocks as a consequence of feeding high grains and low fiber content causing ruminal fermentation disorders and other changes which affect badly the daily gain and finally the cattle industry. So the monitoring groups of bullock for signs of the condition are now crucial. On the basis of pH values the animals were classified into group 1 (animals with rumen pH>5.7) and group 2 (animals with rumen pH<5.6).The consequences of SARA are diverse and complex. Diarrhoea and laminitis are regularly connected to SARA and the adverse impact of organic acids on the ruminal wall which lead to erosion and inflammation enabling translocation of pathogens into the bloodstream provoking inflammation and abscessation throughout the ruminants body. The prevention of SARA includes the establishment of feeding and management manners seeking to minimize the rumen acidotic load. Regular monitoring may facilitate early recognition of the condition and limit the economic losses.
Keywords | SARA, Incidence, Laminitis, Liver abscesses
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 26, 2016; Accepted | September 28, 2016; Published | October 07, 2016
Correspondence | Noura E Attia, Lecturer of Internal Medicine, Animal Medicine Department.,Faculty of Veterinary Medicine, Zagazig University, Zagazig - 44511, Egypt; Email: noura_abobaker@yahoo.com
Citation | Attia NE (2016). Subacute ruminal acidosis in feedlot: Incidence, clinical alterations and its sequelae. Adv. Anim. Vet. Sci. 4(10): 513-517.
DOI | Http://dx.doi.org/10.14737/journal.aavs/2016/4.10.513.517
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Attia. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Subacute ruminal acidosis (SARA) is a common nutritional disease and the economically important problem affects feedlot as well as dairy cattle (Plaizier et al., 2012) Subacute ruminal acidosis is a health and welfare issue as consequently causing the secondary symptoms that occur in response to the digestive upsets which impact significantly on the herd (Hall and Averhof, 2000; Oetzel, 2003).
Subacute ruminal acidosis occurs when high amounts of carbohydrates are introduced to cattle which are normally adapted to digest predominantly forage diets (Oetzel, 2003) or when feedlot cattle without proper adaptation are rapidly transitioned from roughage to high concentrate diets (Beauchemin and Penner, 2009). The disease is characterized by ruminal fluid pH below 5.6 for more than 3 h/24 h (Plaizier et al., 2008) due to the increased production of volatile fatty acids (VFA) and insufficient ruminal buffering (Plaizier et al., 2012).
The financial impacts of SARA on a herd are often insidious and frequently go un-noticed. Financial losses caused by SARA result from reduced body weight gain, plus additional losses from liver abscesses formation and treatment costs of the disease sequelae. Subacute ruminal acidosis is most commonly developed under intensive livestock production systems (Krause and Oetzel, 2006) as feedlots farmers tend to push the grain content in the diets to an even higher level in order to increase the body weight gain. Processing of grains introduced to the animals to increase palatability and the digestibility of starch aggravates the condition.
Subacute ruminal acidosis diagnosis is a difficult task, due to lack of pathognomonic signs as the development of SARA is not associated with early clinical symptoms (Nagaraja and Titgemeyer, 2007; Wahrmund et al., 2012). The correct diagnosis of SARA depends mainly on pH of the
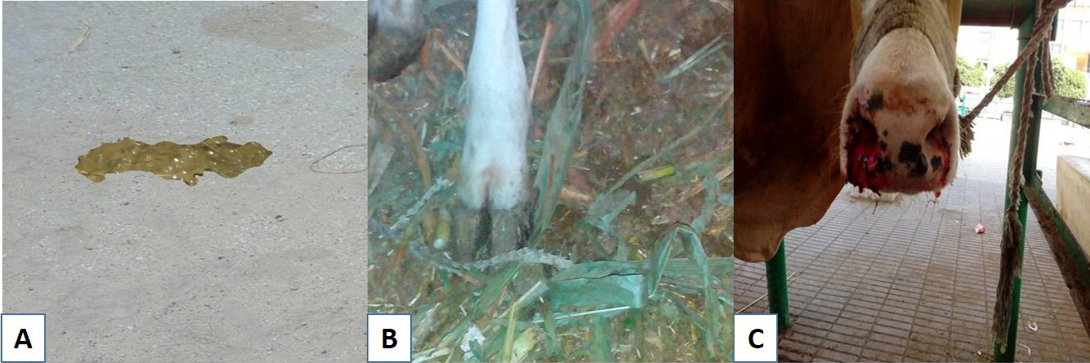
Figure 1: Subacute ruminal acidosis sequelae: A) soft green yellowish feces; B) redness in the interdigital space; C) Nasal bleeding
ruminal fluid, milk fat depression in dairy cattle and reduction in urinary pH and these symptoms must be associated with the herd’s history (Enemark, 2009).
So, the present study was aimed for studying of SARA in feedlots using pH measurements, Incidence, clinical findings and its sequelae of the disease in commercial farms.
MATERIALS AND METHODS
Ethical Consideration
The study protocol was approved by the Ethics of committee of Egyptian Veterinary Medicine Authority.
Animals
This study was conducted on two fattening farms of a total number of 185 bullocks in Ismailia Governorate (Salhia City). The selection of these farms was according to the history which included repeated cases of diarrhoea, laminitis, bloat as well as liver abscesses at slaughter. From these farms a total of 85 fattening bullocks were selected randomly. Twenty- five in the beginning of fattening and 60 in the finisher phase. The production data and herd features were recorded. The study was carried out from May to August 2015. The bullocks were kept on concentrated ration during the period of fattening with an addition to hay or silage 12% and 8% in starting and finishing rations respectively. The animals were fed ad. Lib. Animals were housed in groups under the cover of feedlot system. Clean fresh water was always available for all animals. Additionally, 10 healthy animals were included in this study as a control.
Clinical Assessment
All animals were subjected to thorough clinical examination according to the method described by Dirksen et al. (1990). Assessment of the health status of the bullocks by eye appearance (conjunctival mucous membranes and sclera blood vessels), body temperature, respiration rate, heart rate and ruminal movement were recorded.
Ruminal juice was collected using stomach tube and assessed for saliva contamination. The first jets of sample were discarded to avoid the effect of saliva. Ruminal fluid samples were collected for detection of pH immediately with a portable pH-meter (Horiba, B-213, Kyoto, Japan) and applying of physical examinations (colour, smell, consistency and activity of ruminal protozoa). Ruminal pH measured twice daily 2-4 h after concentrate feeding as recommended by Chaidate et al. (2014). Urine pH was determined (from the mid-stream samples) using urine strips according to the directions of the manufacturer (Roche combur urine strips®, Boehringer Monnheim, Germany). On the basis of pH values obtained the animals were classified into group 1 that have a ruminal pH>5.7 “Non SARA affected group” and group 2 that have a ruminal pH<5.6 “SARA affected group”.
Abattoir Survey
Animals were slaughtered when they reached the required body weight, the slaughtered bullocks were examined for the presence of ruminal lesions and liver abscesses.
RESULTS
The incidence of SARA in the examined farms was high ranged from 32.5-37.7% as illustrated in Table 1. Clinical examination of SARA affected animals not extremely differed than that of the control group. SARA has not characteristic signs but the herd was suffering from cyclic feed intake, depression in some cases, the feces are bright yellowish in colour (Figure 1A), have a sweet–sour smell and contain undigested feed particles, laminitis and bloat were recorded, furthermore epistaxis was found in one case.
Table 1: Incidence of SARA in the examined farms
|
Farm No. |
Population |
No. of examined bulls |
Ruminal pH |
Incidence of SARA |
|
|
>5.7 |
<5.6 |
||||
|
1 |
90 |
40 |
27 |
13 |
32.5% |
|
2 |
95 |
45 |
28 |
17 |
37.77% |
Physical characters of the ruminal juice of SARA affected animals were changed as illustrated in Table 2. The most predominant change was odor of the fluid “sour” and pH “less than 5.6”.
Table 2: Most important changes in rumen fluid in SARA affected animals
|
Variable |
Normal |
SARA affected animals |
|
Colour |
Grey-brown or Green |
Slightly milky/ brown |
|
Odour |
Aromatic |
Sour |
|
Viscosity |
Slightly Viscous |
Slightly viscous |
|
Flotation / Sedimentation |
4–8 min |
No |
|
pH |
5.7–6.8 |
<5.6 |
Complicated sequelae of SARA were shown in the Table 3, the most common sequelae was diarrhoea and laminitis (Figure 1B) then bloat and the least common one was pulmonary hemorrhage (Figure 1C).
Table 3: The percentage of complicated sequelae of SARA
|
Sequelae of SARA |
No of affected animal |
Percentage |
|
Diarrhoea |
9 |
30% |
|
Laminitis |
7 |
23.33% |
|
Bloat |
8 |
26.66% |
|
Pulmonary hemorrhage |
1 |
3.33% |
|
Abomasal displacement and abomasal ulcer |
0 |
0% |
At abattoir, liver abscesses were recorded sometimes separately (Figure 2) and sometimes with ruminal lesions as recorded in Table 4.
Table 4: The number of abscessed livers and ruminal lesions separately or together in slaughtered fattening bullocks “total samples 30”
|
Samples |
Number |
|
Liver abscesses |
7 |
|
Ruminal lesions |
5 |
|
Liver abscesses& Ruminal lesions |
3 |
DISCUSSION
High incidence of SARA in the examined farms may be attributed to the high concentrate ration which contains only 8-12% roughage, fine grinding of the ration and poor feeding management (Nordlund et al., 1995). Selection of the farms participated in this study was done according to the history of the presence of diarrhoea, laminitis, decrease daily gain, bloat and liver abscesses at slaughter. These signs are indicative for the presence of SARA in the herds (Plaizier et al., 2012).
Cyclic feed intake may be attributed to low ruminal pH, increased the osmolality of the ruminal contents due to the excessive production of lactic acid, which inhibits feed intake (Carter and Grovum, 1990). Rumenitis (inflammation of the ruminal epithelium) could also play a role in depressing feed intake. Alteration in animal attitude “depression’ may be due to the pain caused by inflammation of the ruminal epithelium.
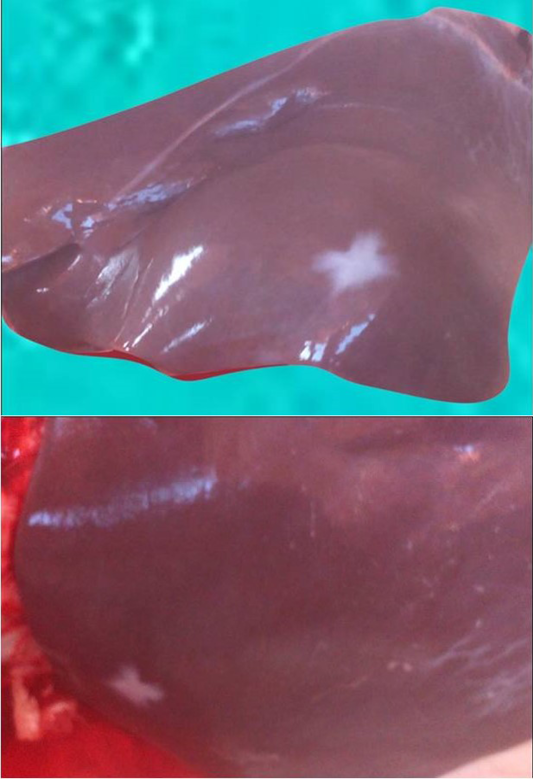
Figure 2 Multiple hepatic abscesses
Similar fecal characters in SARA affected animals were previously described by Kleen et al. (2003) and Bramley et al. (2012). Intermittent diarrhoea and the presence of undigested particles in fecal matter indicate inadequate digestion and fast passage of feed. Changes in the ruminal fluid samples obtained from SARA affected bullocks are attributed to the produced lactic acid.
SARA Sequelae
Diarrhoea is the most common sequelae in the herd with SARA and this may be attributed to post-ruminal fermentation in the intestines due to the excessive outflow of highly fermentable carbohydrates from the rumen (Oetzel, 2000). Another an explanation could be the high osmolarity, which is described for the ingesta in SARA-affected animals, lead to soft feces, due to the increased fluid in the intestinal lumen (Garry, 2002).
Laminitis was present in the herd in 23.33%. Laminitis (hoof discoloration, sole hemorrhage and sole ulceration) may be attributed to the endotoxin released intraruminally from death and lysis of microflora as a consequence of lowering ruminal PH. The endotoxin is absorbed into the blood circulation, inducing a vascular reaction (Mathew and Ajithkumar, 2014).
Bloat recorded in some cases may be due to low ruminal pH which decreases ruminal movement causing an accumulation of gas in the rumen and reticulum. Additionally, frothy bloat is caused by entrapment of gas produced from fermentation of readily digestible feeds (high digestible legumes or cereals). Slime (extracellular bacterial mucopolysaccharides) increases the viscosity of ruminal fluid leading to trapping gases and forming the stable foam that causes the bloat (Cheng et al., 1998).
From rare sequelae of SARA is caudal vena cava syndrome which cause hemoptysis “pulmonary hemorrhage’ and per acute deaths due to massive pulmonary hemorrhage in animals that are affected with SARA (Nordlund et al., 1995). In these cases, septic emboli from liver abscesses lead to lung infections which ultimately invade pulmonary vessels and cause their rupture (Rebhun, 1995).
Liver abscesses which observed at slaughter house occurred as ruminal epithelial cells are not protected by mucus, so they are prone to chemical damage by acids. Low ruminal pH due to the massive production of volatile fatty acids and lactic acid leads to rumenitis, erosion and ulceration of the ruminal epithelium. Once the ruminal epithelium inflamed, the bacteria may colonize the ruminal papillae and spread into the portal circulation causing hepatic abscesses.
Urine pH was decreased in SARA affected bullocks compared with the control ones. Some authors believe that routine measuring of the urine pH is indicative for prediction of SARA in the herd (Enemark et al., 2002). The use of urine pH in the diagnosis of SARA has been doubted (Kleen, 2004), although, it is recommended by other authors (Enemark et al., 2002), as it is one of the most efficient diagnostic parameters.
To minimize SARA risk and its sequelae is to provide sufficient time for the rumen environment to adapt the dietary changes (Schwartzkopf-Genswein et al., 2003). This adaptation time allows the ruminal epithelium and the rumen microbial populations to acclimate the changes in substrate supply.
CONCLUSION
Subacute ruminal acidosis is a substantial health and an important nutritional problem in ruminants. The major predisposing factor is malpractice in feeding. The presence of diarrhoea, laminitis or bloat may strongly indicate that the herd suffering from SARA. Repeated rumen pH measurement “twice daily 2-4 h after concentrate feeding” remains the first choice for diagnosing SARA. Colour, consistency, odour of rumen fluid and the activity of ruminal flora gives the clues for diagnosis. Feedlots require specifically formulated adaptation rations (roughage percentage is decreased and concentrate percentage is increased gradually) over a period of at least 3 weeks.
Acknowledgements
I would like to thank all Veterinary doctors in El- Salhia Farms, Ismalia province, Egypt for their support during conduction of this work.
Conflict of Interest
The author declares no conflict of interest.
References






