Advances in Animal and Veterinary Sciences
Research Article
Clinical Investigation of the Pathogenicity of Pasteurella multocida Isolated from Cattle in Egypt Regarding its Effect on Hematological, Biochemical, and Oxidant-Antioxidant Biomarkers as well as Proinflammatory Cytokines and Acute Phase Proteins
Tamer S. Allam1*, Lamiaa Said1, Mohamed Sabry Abd Elraheam Elsayed2, Nahed Saleh1
1Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Monufia 32897, Egypt; 2Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Monufia 32897, Egypt.
Abstract | This study aimed to screen Pasteurella multocida for different virulence genes, antimicrobial resistance profiles, and to examine its effect on hematological, biochemical, oxidant-antioxidant biomarkers, proinflammatory cytokines, and acute-phase proteins in infected cattle. A total of 70 cattle from the Monufia and Qalyubia governorates included 30 animals suffering from respiratory manifestations and 40 cases were apparently healthy. P.multocida was detected in diseased animals and represented 30/70(42.9%) as confirmed by PCR. Capsular typing confirmed that types A and D represented 10/30(33.3%) and 20/30(66.7%) of the isolates, respectively. The distribution of ompA, sodC, toxA genes encoding outer membrane protein A, superoxide dismutase, and dermonecrotic toxin, respectively, were detected at frequencies of 28/30(93.3%), 22/30(73.3%), and 5/30(16.7%). The most effective antimicrobials were ciprofloxacin, amoxicillin/clavulanic acid, chloramphenicol, and azithromycin. P.multocida isolates were categorized to 14 groups after their antimicrobial resistance profiles, the antimicrobial resistance indices ranged from 0.0 to 1.0. Hematological changes included a significant reduction in RBCs, Hb, PCV, MCV, MCH, MCHC, and lymphocytes, in addition to significant leukocytosis, granulocytosis, and monocytosis. Biochemical alterations included a significant decrease in serum albumin, albumin/globulin ratio, sodium, calcium, phosphorus, chloride, iron, and TIBC concentrations with a significant increase in serum ALT, AST, TP, globulin, creatinine, urea, and potassium. Alterations in oxidant-antioxidative status included a significant increase in serum MDA and NO with a significant decrease in serum TAC, GSH, and SOD. An exaggerated response of proinflammatory cytokines (IL-1, IL-6, TNF-α) and acute-phase proteins (SAA, Hp) was observed. Hence, this work may assist in clarifying the pathogenesis of the disease and thus inform efficient diagnosis and treatment.
Keywords | Pasteurella multocida, Antimicrobial resistance, Acute-phase response, Biochemistry, Hematology
Received | January 09, 2021; Accepted | January 28, 2021; Published | April 01, 2021
*Correspondence | Tamer S Allam, Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Sadat City, Sadat City, Monufia 32897, Egypt; Email: tamer.salah@vet.usc.edu.eg
Citation | Allam TS, Said L, Elsayed MSAE, Saleh N (2021). Clinical investigation of the pathogenicity of pasteurella multocida isolated from cattle in egypt regarding its effect on hematological, biochemical, and oxidant-antioxidant biomarkers as well as proinflammatory cytokines and acute phase proteins. Adv. Anim. Vet. Sci. 9(6): 792-801.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.6.792.801
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Allam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Pasteurella multocida is a zoonotic Gram-negative bacterium of global distribution associated with economic losses in animal production (Steen et al., 2010). It causes hemorrhagic septicemia, fowl cholera, atrophic rhinitis, and snuffles in bovids, birds, swines, and rabbits, respectively (Moustafa et al., 2015). Hemorrhagic septicemia is a highly fatal disease in buffalo and cattle (Orynbayev et al., 2019). The virulence arsenal of P.multocida includes capsular antigens and virulence genes (Katsuda et al., 2013), which are vital in bacterial functionality, protective immunity, vaccine development, and pathogenesis (Hatfaludi et al., 2010). Genes controlling the virulence of P.multocida comprise adherence and colonization factors (ptfA, fimA, hsf-1-2, pfhA, and tadD), iron-regulated and acquisition proteins (exbB,D, tonB, hgbA,B, and Fur), hyaluronidase (pmHAS), superoxide dismutases (sodA,C, and tbpA), neuraminidase (nanB and nanH), lipopolysaccharides (LPS), toxins (toxA);various outer membrane proteins, such as protectins (ompA,omph,oma87, and plpB), and capsule proteins (Katoch et al., 2014). Antimicrobial resistance is of great concern, resulting in prolonged illness, increased treatment time, augmented treatment costs, and occasionally animal mortality (WHO, 2014). Although several studies have been conducted on P.multocida, there is scarce information about its pathogenicity and its effect on hematological, biochemical, and immunological parameters. Therefore, to overcome this gap, this work aims to isolate and identify P.multocida from healthy and pneumonic cattle to perform molecular confirmation, capsular typing, detection of virulence genes, and display of antimicrobial susceptibility profiles of the gained isolates. Moreover, to investigate changes in hematology, biochemistry, biomarkers of oxidative stress, with particular emphasis on the role of proinflammatory cytokines and acute-phase proteins in disease control.
MATERIAL AND METHODS
Animals
Seventy cattle (age range, 10 days to 24 months) from different cities in the Monufia and Qalyubia governorates, Egypt were included. Thirty animals were suffering from pneumonia with nasal and ocular discharges, tachypnea, coughing, and abnormal lung sound on auscultation; these animals comprised the diseased group. The remaining apparently healthy animals (n = 40) served as the control group.
Bacteriological Samples
Seventy nasal swabs were collected from both the diseased and apparently healthy cattle. The swabs were collected in brain heart infusion broth (Difco Laboratories) with 5 mg/L of clindamycin phosphate (Upjohn), 0.75 mg/L of gentamicin sulfate (Roussel Laboratories), 2.5 mg/L of potassium tellurite (BDH), and 5 mg/L of amphotericin B (Sigma–Aldrich). The swabs were transferred on ice to the Department of Bacteriology, Mycology, and Immunology, Faculty of veterinary Medicine, University of Sadat City. Next, the swabs were streaked onto brain heart infusion agar, with 5% sheep blood with the same broth additives, and incubated at 37oC for 48 h (Knight et al., 1983).
Identification of Isolates
Hemolytic activity on blood agar and growth on MacConkey agar were evaluated. Biochemical identification was performed using indole production, methyl red, Voges–Proskauertest, urease activity, H2S production, nitrate reduction, citrate utilization, lysine and ornithine decarboxylase activities, and acid production from arabinose, dulcitol, glucose, galactose, inositol, lactose, maltose, mannitol, mannose, salicin, sucrose, sorbitol, trehalose, and xylose sugars (Pillai et al., 2013).
Molecular Characterization of P.multocida Isolates and Virulence Markers
DNA was extracted using the QIAamp Miniprep Kit (Qiagen) according to the manufacturer’s instructions. The primers for KMT1, capsular typing genes (hyaD-hyaC type A and dcbF type D), and virulence genes ompA, sodC, and toxA were synthesized by Applied Biosystems (Foster City, California) and were implemented in simplex PCR assays in total reaction volume of 25 μL, containing 12.5 μL of Emerald Amp® GT PCR master mix (Takara, Japan), 1 μL forward primer (20 pmol),1 μL reverse primer (20 pmol), 2 μL template DNA, and 8.5 μL of PCR-grade water (Table 1).
Antimicrobial Susceptibility Testing
The disk diffusion method was applied, using the following antibiotic disks (Oxoid): amikacin, amoxicillin/clavulanic acid, azithromycin, cefotaxime, chloramphenicol, and ciprofloxacin. The interpretation of the inhibition zones was performed according to CLSI (2013), and the antimicrobial resistance index was calculated according to (Elsayed et al., 2018).
Blood Samples
Blood samples were collected from the jugular vein under aseptic conditions. The samples were divided into two parts. The first part was collected in disodium ethylene diamine tetra-acetic acid tubes for hematological assays. The second part was collected in plain centrifuge tubes for separation of serum. The serum samples were sectioned into aliquots in Eppendorf tubes so that each was used one time, and the tubes were stored at -20°C until used for analyzing biochemical, oxidant-antioxidant parameter, acute phase proteins, and proinflammatory cytokines.
Hemogram
Hematological parameters included red blood cell count (RBCs), hemoglobin concentration (Hb), packed cell volume (PCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), total leukocyte count,
Table 1: Primers, sequences, and amplicon sizes
|
Target gene
|
Primer sequence (5'-3') | Annealing temperature | Amplicon size | Reference |
| KMT1 | ATCCGCTATTTACCCAGTGG GCTGTAAACGAACTCGCCAC | 55 | 460 | |
|
hyaD-hyaCtype A |
CATTTATCCAAGCTCCACC GCCCGAGAGTTTCAATCC | 55 | 760 | |
|
dcbf type D |
TTACAAAAGAAAGACTAGGAGCCC CATCTACCCACTCAACCATATCAG | 55 | 657 | |
|
OmpA |
CGCATAGCACTCAAGTTTCTCC CATAAACAGATTGACCGAAACG | 55 | 201 | |
|
toxA |
CTTAGATGAGCGACAAGG GAATGCCACACCTCTATAG | 55 | 864 | |
|
sodC |
AGTTAGTAGCGGGGTTGGCA TGGTGCTGGGTGATCATCATG | 55 | 235 |
Table 2: Origin, biochemical identification and molecular confirmation of P.multocida isolates
| Groups | Diseased (30) | Apparently healthy (40) | |||
| Origin | Monoufiya | Qaliobyyia | Monoufiya | Qaliobyyia | |
| Number | 25 | 5 | 32 | 8 | |
|
Pasteurella multocida isolation |
+ve 30/30(100%) |
25/30 (83.3%) |
5/30 (16.7%) |
0 (0.0%) |
0 (0.0%) |
|
-ve 40/40(100%) |
0 (0.0%) |
0 (0.0%) |
32/40 (80%) |
8/40 (20%) |
|
| Biochemically confirmed |
+ve 30/30(100%) |
25/30 (83.3%) |
5/30 (16.7%) |
0 (0.0%) |
0 (0.0%) |
| KMT1 | 30/30(100%) |
25/30 (83.3%) |
5/30 (16.7%) |
0(0.0%) |
0 (0.0%) |
| hyaD-hyaC type A | 10/30(33.3%) |
10/10 (100%) |
-------- | -------- | -------- |
|
Dcbf type D |
20/30(66.7%) |
15/20 (70%) |
5/20 (25%) |
0 (0.0%) |
0 (0.0%) |
Table 3: Molecular confirmation, virulence genes, and antimicrobial susceptibility profiles of 30 confirmed isolates
| Groups | Number of contained isolates | Capsular types | Virulence genes | Antimicrobial resistance patterns | MAR index | |||
|
dcbf type D |
hyaD-hyaC type A |
toxA |
sodC |
omp A |
||||
| 1 | 2 |
2/2 (100%) |
0/2 (0.0%) |
0/2 (0.0%) |
2/2 (100%) |
2/2 (100%) |
AK,AMC,AZM,CTX. | 0.67 |
| 2 | 2 |
0/2 (0.0%) |
2/2 (100%) |
2/2 (100%) |
2/2 (100%) |
2/2 (100%) |
AK,AZM,CTX,C. | 0.67 |
| 3 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
1/1 (100%) |
AK,AZM,C. | 0.5 |
| 4 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
1/1 (100%) |
AMC,CTX. | 0.33 |
| 5 | 5 |
5/5 (100%) |
0/5 (0.0% |
3/5 (60%) |
3/5 (60%) |
5/5 (100% |
AK,AZM,CTX. | 0.5 |
| 6 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
1/1 (100%) |
AK,AMC,AZM,CTX,C,CIP | 1 |
| 7 | 1 |
1/1 (100%) |
0/1 (0.0% |
0/1 (0.0% |
1/1 (100%) |
1/1 (100%) |
AK,AMC,CTX. | 0.5 |
| 8 | 8 |
0/8 (0.0% |
8/8 (100%) |
0/8 (0.0% |
8/8 (100%) |
8/8 (100%) |
AK,CTX. | 0.33 |
| 9 | 1 |
1/1 (100%) |
0/1 (0.0% |
0/1 (0.0% |
1/1 (100%) |
1/1 (100%) |
Not resistant | 0.0 |
| 10 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
1/1 (100%) |
AK,AMC,AZM,CTX,C. | 0.83 |
| 11 | 3 |
3/3 (100%) |
0/3 (0.0%) |
0/3 (0.0%) |
0/3 (0.0%) |
3/3 (100%) |
AK,CTX,C. | 0.5 |
| 12 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
AZM,CTX. | 0.33 |
| 13 | 2 |
2/2 (100%) |
0/2 (0.0%) |
0/2 (0.0%) |
0/2 (0.0%) |
0/2 (0.0%) |
AK,CTX,CIP. | 0.5 |
| 14 | 1 |
1/1 (100%) |
0/1 (0.0%) |
0/1 (0.0%) |
1/1 (100%) |
1/1 (100%) |
AK,AZM,CTX,CIP. | 0.67 |
|
Total |
30 |
20/30 (66.7%) |
10/30 (33.3%) |
5/30 (16.7%) |
22/30 (73.3%) |
28/30 (93.3%) |
||
and differential leukocyte counts. The hematological parameters were assessed automatically by a BeneSpheraTM Brand3 Part differential veterinary hematology analyzer H23 (Avantor Performance Materials, Inc, The Netherlands, Holanda, Model: H23vet. Serial No: 931716004) and according to Feldman et al. (2000).
Serum Biochemical Parameters
The evaluated serum biochemical parameters included total protein (TP) [Bio Med, Germany], albumin (Alb), urea, alanine aminotransferase (ALT), aspartate aminotransferase (AST), iron and total iron-binding capacity (TIBC) [Biodiagnostics, Egypt], along with creatinine, calcium (Ca), inorganic phosphorus (ip), sodium (Na), potassium (K) and chloride (Cl) [Spectrum, Germany]. These parameters were investigated colorimetrically with following the instructions of the manufacturers. The serum globulin was determined by subtracting values of total serum protein from that of serum albumin as well as the ratio between albumin and globulin concentrations was also calculated according to Bobbo et al. (2017).
Oxidant And Antioxidant Parameters
Oxidative stress was investigated by measuring serum malondialdehyde (MDA), nitric oxide (NO), reduced glutathione (GSH), superoxide dismutase (SOD), and total antioxidant capacity (TAC). These parameters were evaluated colorimetrically with following the instructions of the manufacturer (Biodiagnostics, Egypt).
Proinflammatory Cytokines And Acute-Phase Proteins
Serum levels of proinflammatory cytokines including interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were measured by enzyme-linked immunosorbent assays using commercial kits (Shanghai Coon Koon Biotech Company, China). Acute-phase proteins (APPs) included serum haptoglobin (Hp) and serum amyloid-A (SAA), which were evaluated by spectrophotometer using commercial kits (Boehringer Ingelheim, Germany) with following the manufacturers’ instructions.
Statistical Analysis
Values were presented as means ± standard deviations. Differences were evaluated statistically using Student’s t test, as implemented in Statistical Package for Social Sciences v.16 released in 2007, and considered statistically significant at probability (P) values <0.05.
RESULTS
Isolation, Biochemical Identification, Molecular Confirmation, And Antimicrobial Sensitivity Profiles Of P.multocida
Of the 70 nasal swabs, 30(42.9%) from the diseased animals exhibited growth on P.multocida-specific medium, while the 40(57.14%) obtained from apparently healthy cattle exhibited no growth (P˂0.05; Table 2). All the
Table 4: Hematological parameters
| Parameters | Group (N=30) | |
| Apparent healthy | Diseased | |
|
RBCs (x106/µl) |
7.91±1.29a |
4.7±0.41b |
| Hb (g/dl) |
12.66±1.56a |
8.41±1.05b |
| PCV (%) |
31.9±3.60a |
20.31±2.28b |
| MCV (fl) |
36.94±1.70a |
32.75±0.65b |
| MCH (pg) |
23.03±2.15a |
18.4±0.93b |
| MCHC (%) |
53.99±5.72a |
44.76±1.64b |
|
TLC (x103/µl) |
10.46±1.50b |
19.28±4.64a |
|
Granulocyte (x103/µl) |
4.93±1.29b |
9.35±1.79a |
|
Lymphocyte (x103/µl) |
4.92±0.84a |
2.58±0.75b |
|
Monocytes (x103/µl) |
1.78±0.63b |
3.66±0.43a |
N: number of animals in each group
Values are mean ± SD. Values followed by different superscript litters in the same row are differ significantly at p<0.05.
Table 5: Serum biochemical, oxidant and antioxidant parameters, acute phase proteins and proinflammatory cytokines
| Parameters | Group (N=30) | |
| Apparent healthy | Diseased | |
| TP (g/dl) |
6.09±0.13b |
6.94±0.24a |
| Albumin (g/dl) |
4.12±0.33a |
3.12±0.23b |
| Globulin (g/dl) |
1.97±0.34b |
3.82±0.36a |
| Albumin globulin ratio |
2.17±0.53a |
0.83±0.13b |
| ALT (U/L) |
23.15±0.66b |
27.93±1.08a |
| AST (U/L) |
37.83±1.16b |
46.76±1.37a |
| Urea (mg/dl) |
27.89±2.18b |
40.59±3.98a |
| Creatinine (mg/dl) |
0.82±0.30b |
1.57±0.18a |
| Na (mmol/L) |
420.21±52.55a |
292.89±18.51b |
| K (mmol/L) |
3.46±0.29b |
6.12±0.65a |
| Cl (mmol/L) |
96.23±0.75a |
89.66±1.52b |
| Ca (mg/dl) |
12.38±0.21a |
10.41±0.44b |
| P (mg/dl) |
4.59±0.53a |
3.57±0.31b |
| Serum iron (μg/dl) |
145.20±10.85a |
114.44±6.90b |
| TIBC (μg/dl) |
293.33±12.05a |
223.81±9.75b |
| NO (μmol/L) |
71.0±6.56b |
87.67±5.13a |
| GSH (mmol/L) |
1205.0±343.27a |
712.0±234.34b |
| SOD (U/L) |
141.0±18.19a |
128.0±1.0b |
| TAC (mM/L) |
0.63±0.36a |
0.21±0.02b |
| MDA (nmol/mL) |
1.50±0.45b |
2.17±0.15a |
| SAA (mg/L) |
1.43±0.15b |
4.17±1.29a |
| HP (mg/dl) |
63.33±4.16b |
76.67±5.51a |
| IL-1 (pg/ml) |
14.1±2.81b |
23.71±9.32a |
| IL-6 (pg/ml) |
31.22±4.99b |
46.92±7.33a |
|
TNF-α (pg/ml) |
7.70±0.93b |
11.04±1.81a |
N: number of animals in each group
Values are mean ± SD. Values followed by different superscript litters in the same row are differ significantly at p<0.05.
obtained isolates 30/30(100%) were biochemically identified as P.multocida, and all of them were confirmed by PCR (Table 2; Figures 1 and 2). Most of the isolates harbored the dcbf type D capsular gene 20/30(66.7%), while 10/30(33.3%) exhibited the hyaD-hyaC type A capsular gene (P˂0.05; Tables 2 and 3; Figures 1 and 2). The majority of the isolates (28/30, 93.3%) tested positive for the virulence gene ompA (P˂0.05; Table 3; Figures 1 and 2). Moreover, 22/30(73.3%) harbored sodC, and a minority of the isolates (5/30, 16.7%) expressed toxA (P˂0.05; Table 3; Figures 1 and 2). Furthermore, all the 30 P.multocida isolates could be categorized into 14 groups (1-14) according to antimicrobial resistance profiles. The most effective antimicrobials were ciprofloxacin, amoxicillin/clavulanic acid, chloramphenicol, and azithromycin. The multiple antimicrobial resistance indices ranged from zero to one, with a significant difference between some of the dissimilar indices (P˂0.05; Table 3).
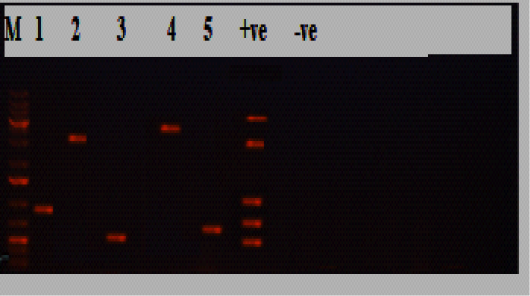
Figure 1: Electrophoresis results of P.multocida isolate from the diseased group, Lane M: marker, Lane 1:KMT1 at 460bp, lane 2: dcbf type D at 657bp, lane 3: OmpA at 201bp, lane 4: toxA at 864bp, lane 5: sodC at 235bp, while lane +ve representing internal positive control, and lane 6 was E. coli as negative control.
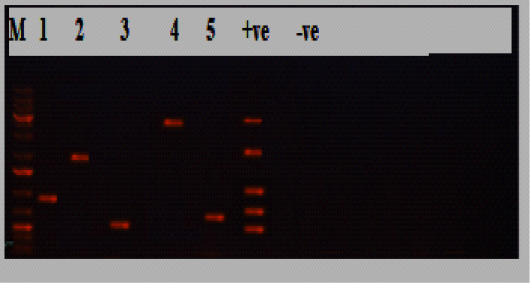
Figure 2: Electrophoresis results of P.multocida isolate from the diseased group, Lane M: marker, Lane 1:KMT1 at 460bp, lane 2: hyaD-hyaCtype A at 760bp, lane 3: OmpA at 201bp, lane 4: toxA at 864bp, lane 5: sodC at 235bp, while lane +ve representing internal positive control, and lane 6 was E. coli as negative control.
Hematology
Diseased animals exhibited a significant reduction in RBCs, Hb, PCV, MCV, MCH, and MCHC values compared with the apparently healthy animals (P <0.05; Table 4). Moreover, a significant increase in WBC, granulocyte, and monocyte levels was detected in diseased cattle (P<0.05) with a significant decrease in lymphocyte levels (P <0.05; Table 4).
Serum Biochemistry
There was a significant decrease in serum albumin, albumin/globulin ratio, sodium, calcium, phosphorus, chloride, iron, and TIBC levels in the diseased animals (P<0.05; Table 5). Moreover, a significant increase in levels of serum hepatic enzymes (ALT and AST) was observed, in addition to an increase in the concentrations of serum TP, globulin, creatinine, urea, and potassium (P<0.05; Table 5).
Oxidant-Antioxidant Status
A statistically significant increase in the mean values of serum MDA and NO in the diseased cattle was observed compared to the apparently healthy group (P<0.05; Table 5). Moreover, a significant decrease in serum TAC, GSH, and SOD levels was detected in the diseased cattle (P<0.05; Table 5).
Proinflammatory Cytokines And Acute-Phase Responses
A significant increase in the concentrations of IL-1, IL-6, and TNF-α was observed in the diseased cattle compared with the apparently healthy group (P<0.05; Table 5). The serum APPs (Hp and SAA) were significantly elevated in the diseased group compared to the healthy one (P<0.05; Table 5).
DISCUSSION
By isolation and identification, P.multocida was identified in 30/70(42.9%) cattle with pneumonic symptoms, whereas none of the healthy animals were culture-positive. These results are in slight contrast with those of Khamesipour et al. (2014), who isolated P.multocida with a rate of 25/219(11.4%) from animals with pulmonary symptoms and 5/114(4.4%) from animals with healthy lungs. The molecular confirmation using the KMT1 primers targeting the gene of an integral component of the membrane of P.multocida (May et al., 2001) confirmed that 30/30(100%) of the isolates were P.multocida. The molecular capsular typing of P.multocida is considered a rapid alternative to the conventional capsular serotyping technique (Townsend et al., 2001). Most of the isolates (20/30, 66.7%) harbored the dcbF type D capsular gene, while the remaining ten isolates harbored the hyaD-hyaC type A capsular gene, these are similar to the findings by Katsuda et al. (2013), who proved the existence of capsular types A and D among P.multocida isolates originating from cattle. The high frequency of the OmpA gene 28/30(93.3%) highlights its important role in stabilizing the cell envelope structure by linking the outer membrane and peptidoglycan layers (Katoch et al., 2014). Although the sodC gene controls superoxide dismutase represented 22/30(73.3%) in isolates was lower than Khamesipour et al. (2014), the dermonecrotoxin-encoding toxA gene represented 5/30(16.7%) was higher than their findings. The susceptibility of P.multocida to amikacin, amoxicillin, chloramphenicol, and ciprofloxacin was also confirmed by Khamesipour et al. (2014). Moreover, susceptibility to azithromycin and cefotaxime was confirmed by Arora et al. (2005). Isolates exhibiting a MAR index >0.2 are of great public health concern and considered to be of high-risk contamination origin (Shehata et al., 2019).
The diseased cattle showed a significant decrease in RBCs, Hb, PCV, MCV, MCH, and MCHC values, that agree with Fraser et al. (2014). This anemia could be classified as microcytic hypochromic anemia or anemia of inflammatory disease (AID) due to underlying disease. Interestingly, the results revealed decreased serum iron and TIBC concentrations, which allied with the findings of Šoltésová et al. (2015), who found a significant decrease in serum iron in sick calves. Notably, increased concentrations of measured IL-1, IL-6, and TNF-α in diseased animals. Inflammatory cytokines, such as IL-6 produced in the liver, increase the level of hepcidin, which in turn prevents the release of iron from iron stores; this is an element in the immunological reaction aiming to reduce microbial utilization of iron (Fry, 2010). These changes may be attributed to the mononuclear phagocytic system, which becomes hyperplastic, trapping free iron, and hence increases iron storage in phagocytic cells after inflammation. Decreases in iron transfer to developing erythroid cells in the bone marrow leads to a reduction in Hb (Aytekin et al., 2011). Other inflammatory cytokines may share in producing anemia, such as IL1, suppress the proliferation of erythroid precursors in the bone marrow or inhibit the production of erythropoietin in the kidney; in addition, TNF-α-mediated damages erythrocyte membranes (Weiss and Goodnough, 2005).
A major increase in WBCs, granulocytes, and monocyte levels was observed in diseased cases, along with a large decrease in lymphocyte levels. This comes in line with Šoltésová et al. (2015), who stated that the increase in WBCs, mainly neutrophils, was a common finding in many diseases as a consequence of inflammatory processes, such as respiratory diseases in the sick calves. Lymphopenia in the diseased cattle was consistent with Aytekin et al. (2011), who attributed lymphopenia to stress response and endogenous release of corticosteroids, which lead to redistribution of recirculating lymphocytes, resulting in their sequestration in the lymphoid tissues instead of entering efferent lymph and blood to participate in the developing inflammation.
Our results showed a large increase in TP, globulin, and a large decrease in albumin and albumin/globulin ratio. Similar finding reported in calves with respiratory disease (Šoltésová et al., 2015), who attributed hyperproteinemia to hyperglobulinemia or increased hepatic synthesis of the positive APPs responding to inflammation. Acute-phase proteins include serum amyloid A and haptoglobin are recognized as inflammatory markers in cattle as they are produced by the liver in response to pro-inflammatory cytokines (Horadagoda et al., 1999; Eckersall and Bell, 2010). The rise in the studied APPs (serum Hp and SAA) in diseased cattle were in line with the results of El-Deeb et al. (2020). However, other studies have reported a limited association between respiratory diseases and HP levels (Wittum et al., 1996; Young et al., 1996). The significance of Hp attributed to it is a powerful bacteriostatic protein and works by restricting free hemoglobin and depriving microorganisms from iron required for their development (Eaton and Toal, 1982). The SAA response may be attributed to its significant role in modulating the immune defense of animals during infection and/or tissue injury (Orro et al., 2011). Baumann and Gauldie (1994) stated that pro-inflammatory mediators reason disturbance in metabolism of liver, stimulating the production of different acute phase proteins (APPs) in the blood stream. This study showed a significant elevation of serum concentrations of IL-1, IL-6, and TNF-α in the diseased cattle. Inflammatory cytokines are produced from the immune cells in response to infection and act as messengers between the infection site and the hepatocytes to induce the synthesis of APPs from the liver, along with their role in initiating the inflammatory surge that leads to lung injury in bovine pasteurellosis (El-Deeb et al., 2020). The reported hypoalbuminemia may be due to albumin is considered a negative acute-phase protein, and frequently and markedly decline during inflammation or due to anorexia. Moreover, hypoalbuminemia may be associated with progression in renal failure (Lang et al., 2018) which supported with increased the renal function parameters, urea and creatinine. The rise in serum urea and creatinine concentrations come similar to findings of Hashem et al. (2018), who confirmed the adverse effect of virulent P.multocida on renal histology of rabbits. The determination of albumin globulin ratio may be used as a sign of liver function (Kaneko et al., 2008). The reduced albumin globulin ratio could be attributed to the reported hypoalbuminemia and/or hyperglobulinemia, due to increased APPs. The raised serum liver enzymes activity (ALT and AST) in diseased cattle complies with Aytekin et al. (2011), who attributed this to the dysfunction of liver, due to hepatic degenerative, and necrotic changes caused by bacterial infection and toxins.
Clearly, the diseased cattle had a significant decrease in serum concentrations of calcium, phosphorus, sodium, and chloride while serum values of potassium were significantly increased. The distortion of serum electrolytes is attributed to an increase in temperature in the acute course of disease and metastatic infection of the liver and kidneys leading to impaired liver and kidney function (Novert, 2002; Hashem et al, 2018). Whereas, serum hyperkalemia could be seen in respiratory diseases mostly if acidosis is present because H+ ions accumulated in the extracellular fluid is exchanging with potassium present in the intracellular fluid (Kaneko et al., 2008). The serum hypocalcemia might be a result of anorexia, decreased intestinal absorption or increased renal excretion (Radostitis et al., 2000). At the same time 40-45% of calcium is protein bound mainly to albumin, so hypoalbuminemia might be a possible cause for this hypocalcemia (Kaneko et al., 2008). The significant hypophosphatemia seemed to be secondary to reduced phosphorus absorption from the gut and reduced phosphorus resorption from the tissues (Orr et al., 1990).
Oxidative stress is an imbalance between the excessive production and removal of free radicals resulting in irreversible cell damage (Rahal et al., 2014). A significant increase in serum MDA and NO concentrations were noted, along with a significant decrease in serum TAC, GSH, and SOD concentrations. These results coincide with findings of El-Deeb and Tharwat (2015). Chirase et al. (2004) attributed the increase in serum MDA to cellular lipid peroxidation, and the decrease of GSH and SOD levels to the consumption of such elements in the protection of cells against oxidative injury by preventing the peroxidation process and production of excess free radical molecules. The increase in serum NO is attributed to its role in reducing harmful inflammation by enhancing and increasing the host’s ability to recognize and interact with bacterial pathogens (Sheridan et al., 2016).
CONCLUSION
The study showed that pneumonic cattle suffering from respiratory distress were confirmed to be infected with P.multocida through biochemical identification and molecular confirmation. They exhibited alterations in hematology, blood chemistry, oxidant-antioxidant status, proinflammatory cytokines, and APPs. These parameters are considered useful tools that could assist in clarifying the pathogenesis of the disease and inform efforts to improve diagnosis, prognosis, and efficient treatment of P.multocida infection.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, as well as this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
AUTHORS CONTRIBUTION
TSA, LS, NS, MSAE conceived and designed the study. TSA, LS, NS executed the experiment and analyzed hematology, blood chemistry, oxidant-antioxidant profiles with acute phase proteins and proinflammatory cytokines. TSA, LS, NS, MSAE executed the experiment and make bacterial isolation, identification and molecular confirmation. All authors interpreted the data, and critically revised the manuscript for important intellectual contents and approved the final version.
REFERENCES






