Advances in Animal and Veterinary Sciences
Research Article
Pathomorphological Studies on the Effect of Aged Garlic Extract in Aflatoxin B1 Induced Liver Damage in Wistar Albino Rats
Sarvesha Krishnappa1, Mayasandra Lakshmikanth Satyanarayana1, Suguna Rao1, Sreenivasa Yathiraj2, Narayana Bhat Shridhar3, Shivalingappa Yamanappa Mukartal4, Saurabh Gupta5, Shoorvir Singh5*, Kuldeep Dhama6, Anuradha Menon Elattuvalappil7
1Department of Pathology, Veterinary College, Hebbal, KVAFSU, Bengaluru- 560 024; 2Veterinary College, Hebbal, KVAFSU, Bengaluru 560 024; 3Department of Pharmacology and Toxicology; 4Department of Veterinary Microbiology, Veterinary College, Hebbal, KVAFSU, Bengaluru-560 024; 5Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; 6Division of Pathology, Indian Veterinary Research Institute (IVRI), Bareilly, Uttar Pradesh; 7ICAR NAE Project, Veterinary College, Bengaluru, India.
Abstract | The aim of the study was to evaluate the hepatoprotective effect of AGE in AFB1 induced liver damage in Wistar albino rats. Liver damage was induced by inoculating AFB1 (4 mg/kg BW) on day 12 of the experiment. AGE was used (200 mg/kg BW) orally, for a period of 28 days against AFB1 induced liver injury. Histopathologically there was periportal and centrilobular necrosis of hepatocytes and bile duct hyperplasia in AFB1 treated rats, however, when AGE was given along with AFB1 the hepatic parenchyma was apparently normal indicating the beneficial effect of AGE in protecting liver against AFB1 induced injury.
Keywords | Aged garlic extract (AGE), Aflatoxin B1 (AFB1), Bile duct hyperplasia, Histhopathology, Aspergillus
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 31, 2016; Accepted | November 25, 2016; Published | December 11, 2016
*Correspondence | Shoorvir Singh, Division of Animal Health, Central Institute for Research on Goats (CIRG), Makhdoom, PO-Farah, Mathura-281122, Uttar Pradesh, India; Email: shoorvir.singh@gmail.com; shoorvir_singh@rediffmail.com
Citation | Krishnappa S, Satyanarayana ML, Rao S, Yathiraj S, Shridhar NB, Mukartal SY, Gupta S, Singh S, Dhama K, Elattuvalappil AM (2016). Pathomorphological studies on the effect of aged garlic extract in aflatoxin B1 induced liver damage in wistar albino rats. Adv. Anim. Vet. Sci. 4(12): 648-654.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.12.648.654
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Krishnappa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Aflatoxin B1 is a metabolite produced by the fungi, Aspergillus flavus and Aspergillus parasiticus, which are contaminants of human and animal grain foods. Corn is probably the most important source of AFB1 for both human and animal consumption (Wood, 1989). AFB1 has been reported to cause acute hepatotoxicity and liver carcinomas in people and laboratory animals (Roebuck and Maxuitenko, 1994). They are metabolized to a highly reactive 8, 9-epoxide that binds to cellular macromolecules, primarily in the periportal region of the liver. AFB1-induced liver injury manifests itself as periportal parenchymal cell necrosis, hemorrhage, and injury to intrahepatic bile ducts. The liver lesions along with loss of appetite and lethargy in exposed animals are collectively referred to as aflatoxicosis.
Garlic is an important medicinal herb and is known to possess scientifically proven medicinal properties. Garlic ranks highly among herbs that help to prevent diseases largely due to its high content of organosulphur compounds and its antioxidant activity. Aged garlic (well ripened garlic stored at room temperature (37°C) for 20 months) extract is a richer source of antioxidants than fresh garlic (Biren et al., 2006). Tadi et al. (1991) reported that the AGE protected liver against toxicity induced by benzopyrene and AFB1, two potent free radical producing environmental carcinogens. It is believed that aged garlic extract exerts hepatoprotective effects through its antioxidant property. Keeping these in view, the present study was undertaken to study the pathomorphological changes in aflatoxin B1 induced liver damage in rats.
Materials and methods
The present study was conducted at the Department of Veterinary Pathology, Veterinary College, Bangalore-560024.
Animals
Wistar albino rats weighing between 150 to 300 g were used for the present study. The animals were obtained from the Central Animal Facility, Indian institute of Sciences, Bangalore 560 012. The study was approved by the Institutional Animal Ethics Committee (IAEC). The animals were fed with standard commercial rat feed obtained from M/S Godrej Agrovet Ltd. Mumbai – 400079.
Aflatoxin B1
To induce hepatotoxicity in rats, single dose of commercially available AFB1 preparation (Sigma-Aldrich Chemicals Ltd.) was used in the present study, which was given intraperitoneally at the dose rate of 4 mg/kg body weight on day 12 of experimental period.
Aged Garlic Extract
Obtained from M/S Vet Care Pvt. Ltd, Yelahanka, Bangalore, and was administered daily at a dose rate of 200 mg/kg body weight, orally through orogastric tube for 28 days.
Experimental Design
In the present study the experimental animals were divided into 4 groups with 6 animals in each group. Group I served as normal control used for studying baseline values of the parameters. Group II (vehicle control) received DMSO (0.3 ml/animal) on day 12 of experimental period. Rats in Groups III and IV were injected with a single dose of AFB1 intraperitoneally after fasting overnight at the dose of 4mg/kg body weight, in 3 per cent DMSO on day 12 of the experimental period. The rats belonging to Group IV were administered AGE suspended in distilled water, orally through orogastric tube at the dose rate of 200 mg/kg body weight from day one to twenty eight of the experimental period. The volume of administered AGE was 1ml for each animal.
Sample Collection
Two animals from each group were sacrificed humanely under anaesthesia on day 14, 21 and 28 of experimental period and a detailed postmortem examination was conducted. Gross lesions were recorded. The tissue samples from all the internal organs were collected on day 14, 21 and 28 of experimental study for recording of weights and histopathological studies. The organs were separated and washed in saline and small pieces were fixed in 10 per cent neutral buffered formalin and processed by routine paraffin embedding. 4-5 µm sections were stained with Haematoxylin and Eosin and examined under microscope. The organ weights were converted into relative organ weights and expressed in percentage as mentioned below:
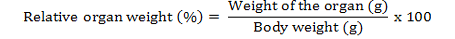
Statistical Analysis
The data generated from the experimental study were subjected to two way analysis of variance (ANOVA) test using Graph Pad Prism version 5 for windows.
Results
A detailed and systematic post mortem examination of the sacrificed animals revealed no appreciable gross changes in liver, kidney, heart, intestines and other organs (Figure 1). All the organs appeared normal except for splenomegaly (Figure 2) which was consistently observed among AFB1 treated animals during each sacrifice.
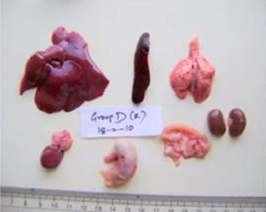
Figure 1: Liver, spleen, kidney, heart, lung, stomach, intestine and pancreas from AFB1+AGE treated rat with normal size, shape and colour on Day 28 of the study
Table 1: Percent relative liver weight of various treatment groups at different time intervals
|
Group |
Days of experimental study |
||
|
Day 14 |
Day 21 |
Day 28 |
|
|
I |
3.52±0.01b (n=2) |
3.41±0.08b (n=2) |
3.44±0.01b (n=2) |
|
II |
3.45±0.04b (n=2) |
3.50±0.02b (n=2) |
3.54±0.01b (n=2) |
|
III |
3.22±0.04a (n=2) |
3.11±0.06a (n=2) |
3.06±0.01a (n=2) |
|
IV |
3.50±0.19b (n=2) |
3.61±0.04b (n=2) |
3.51±0.00b (n=2) |
Means bearing common superscript do not differ significantly at (P≤0.05)
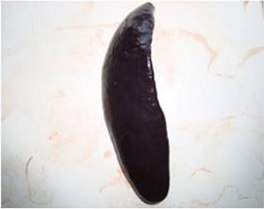
Figure 2: Spleen of AFB1 treated rat showing splenomegaly with rounded border on Day 14 of the study
The mean relative liver weights in AFB1 Group on day 14, 21 and 28 were significantly (P<0.05) lower compared to control Group whereas significantly (P<0.05) higher values were found in DMSO and AFB1+AGE Groups compared to AFB1 Group as shown in Table 1. No significant difference was observed in the relative kidney weight, between treated and control group as shown in Table 2. The mean relative spleen weights in AFB1 Group on day 14, 21 and 28 were significantly (P<0.05) higher compared to control and treatment groups as shown in Table 3.
Table 2: Percent relative kidney weight of various treatment groups at different time intervals
|
Group |
Days of experimental study |
||
|
Day 14 |
Day 21 |
Day 28 |
|
|
I |
0.32±0.01 (n=2) |
0.33±0.01 (n=2) |
0.33±0.01 (n=2) |
|
II |
0.31±0.02 (n=2) |
0.32±0.01 (n=2) |
0.32±0.00 (n=2) |
|
III |
0.31±0.00 (n=2) |
0.33±0.01 (n=2) |
0.32±0.01 (n=2) |
|
IV |
0.32±0.01 (n=2) |
0.31±0.01 (n=2) |
0.31±0.01 (n=2) |
Means bearing common superscript do not differ significantly at (P≤0.05)
Table 3: Percent relative spleen weight of various treatment groups at different time intervals
|
Group |
Days of experimental study |
||
|
Day 14 |
Day 21 |
Day 28 |
|
|
I |
0.52±0.03b (n=2) |
0.55±0.02b (n=2) |
0.54±0.02b (n=2) |
|
II |
0.51±0.02b (n=2) |
0.52±0.01b (n=2) |
0.53±0.01b (n=2) |
|
III |
0.61±0.03a (n=2) |
0.60±0.01a (n=2) |
0.60±0.01a (n=2) |
|
IV |
0.55±0.01b (n=2) |
0.54±0.01b (n=2) |
0.55±0.01b (n=2) |
Means bearing common superscript do not differ significantly at (P≤0.05)
Histologically, the sections of liver on day 14, 21 and 28 showed the normal appearance of hepatic and biliary components among control (Figure 3) and DMSO animals whereas AFB1 treated animals showed swollen, granular appearance of hepatocytes with reduced sinusoidal spaces. In few cases sinusoidal congestion was also observed. The hepatocytes showed degenerative changes ranging from hydropic to fatty change pushing the nucleus to a side. In focal areas fatty changes were very extensive occupying the entire hepatocytes. The hepatic parenchyma showed focal to diffuse areas of necrosis (Figure 4). In addition individual hepatocytes also showed necrosis. The hepatic necrosis was observed both in the portal and periportal area. Centrilobular necrosis was observed prominently in all the cases and in few cases the necrotic area also showed mild heterophilic infiltration. The other changes included increased kupffer cell activity, bile duct epithelial hyperplasia, proliferation of oval and spindle cell in portal tract, nuclear disintegration and chromatolysis.
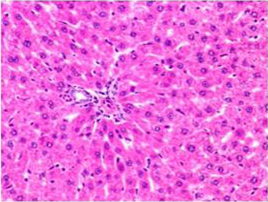
Figure 3: Section of liver from control rat on day 14 showing normal appearance of hepatic parenchyma in the portal area H&E X 100
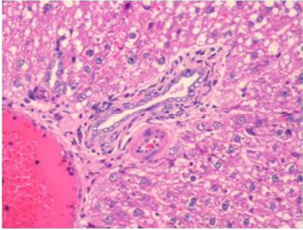
Figure 4: Section of liver from AFB1 treated rat on day 14 showing swollen, granular and vacuolated appearance of cytoplasm of hepatocytes, severe fatty change, periportal and single cell necrosis, bile duct epithelial hyperplasia, nuclear disintegration and Kupffer cell proliferation H&E X 200
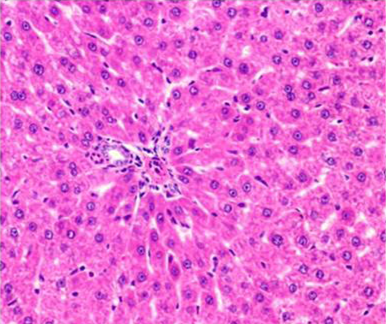
Figure 5: Section of liver from AFB1+AGE treated rat on day 14 showing swollen and granular appearance of hepatocytes around the portal tract H&E X 200
Histologically, the sections of liver from AFB1+AGE treated group showed the mild to moderate lesions viz.; swollen, granular appearance of hepatocytes with reduced sinusoidal spaces (Figure 5), congestion and occasional histiocytic proliferation. Occasional areas showed presence of large and regenerating hepatocytes (Figure 6).
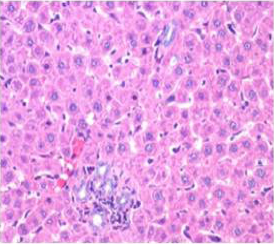
Figure 6: Section of liver from AFB1+AGE treated rat on day 28 showing improved architecture of hepatic parenchyma which includes only mild degree of granularity of hepatocytes and sinusoidal congestion and Kupffer cell hyperplasia H&E X 200
Histologically, the sections of kidney showed the normal appearance of tubular and glomerular structures among control and DMSO fed groups on days 14, 21 and 28 of the experimental period whereas AFB1 treated animals showed swelling, vacuolations, congestion, haemorrhage along with desquamation of tubular and glomerular epithelium (Figure 7). The histological lesions were milder in AFB1+AGE treated group (Figure 8) compared to lesions noticed in AFB1 treated Group.
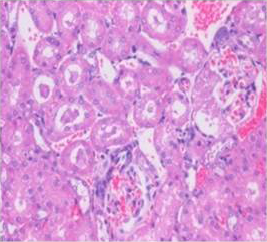
Figure 7: Section of kidney from AFB1 treated rat on day 14 showing swollen and vacuolated appearance of tubular and glomerular epithelium with congestion, haemorrhages, and desquamation of tubular epithelium into the lumen forming hyaline cast H&E X 200
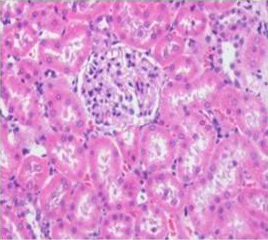
Figure 8: Section of kidney from AFB1+AGE treated rat on day 14 showing slight swollen tubular and glomerular epithelium with congestion H&E X 200
Histologically, the sections of spleen showed the normal distribution of red and white pulp areas among control and DMSO fed animals on day 14, 21 and 28 of the experimental period whereas AFB1 treated animals showed massive congestion (Figure 9), extensive haemorrhagic areas disrupting the white pulp, presence of haemosiderin particles, lymphocytolytic activity and occasional histiocytic activity. In addition, a relative reduction in the white pulp due to massive congestion was noticed among AFB1 Group. Histologicaliy, the sections from AFB1+AGE treated group showed secondary lymphoid follicle formation with mild lymphocytic activity, mild congestion, haemorrhage and occasional histiocytic activity compared to severe lesions noticed in AFB1 Control group (Figure 10). Histologically other organs such as heart, lung, stomach, intestine and pancreas appeared normal in all the groups on different days of observations.
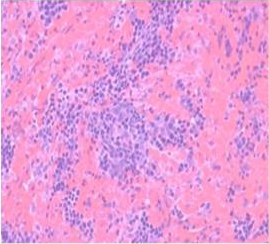
Figure 9: Section of spleen from AFB1 treated rat on day 14 showing massive congestion, extensive haemorrhagic areas disrupting the white pulp and occasional histiocytic activity. Also note relative reduction in the white pulp due to massive congestion H&E X 200
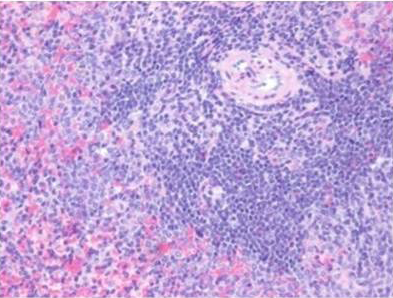
Figure 10: Section of spleen from AFB1+AGE treated rat on day 21 showing secondary lymphoid follicle formation with mild lymphoid necrosis and occasional histiocytic activity H&E X 200
Discussion
In the present study liver damage was induced in rats by administering single dose of AFB1 4 mg/kg body weight by intraperitoneal route on day 12 of the experiment, which was in agreement with Barton et al. (2000) who observed that with a single dose of 4 mg AFB1/kg produced histological changes after 24-48 hrs characterized by pronounced periportal lesions accompanied by hemorrhage that appeared to radiate into the midzonal regions in some rats and also observed swollen parenchymal cells with pyknotic nuclei characteristic of oncotic necrosis. The portal interstitium was separated from parenchyma, suggesting portal edema and biliary epithelial cells appeared to be swollen and undergoing oncotic necrosis.
In the present study, the dose of AGE administered to rats was 200 mg/kg body weight orally for 28 days according to earlier reports of Ezeala et al. (2009) who conducted the study on hepatoprotective effect of fresh garlic extract in acetaminophen induced hepatotoxicity in mice using dose ranging from 250, 500 and 750 mg/kg body weight for five days. AGE is more potent than the fresh garlic extract. In the present study AGE, being more potent than fresh garlic extract was utilized at a dose of 200 mg/kg body weight as the duration of experiment was 28 days.
Significant decrease observed in the relative liver weight in AFB1 treated rats could be attributed to protein derangement, vacuolar degeneration and necrosis of hepatocytes caused by AFB1 administration. This observation was in accordance with that of Manoharan et al. (2008), who recorded significant decrease in the relative liver weight of Wistar rats upon AFB1 administration. The absence of relative decrease in liver weight in AGE treated rats that received AFB1 may be attributed to the fact that AGE protected liver damage is induced by AFB1 by its antioxidant and anti-lipid peroxidative property.
No significant difference was observed in the relative kidney weight between treated and control groups. This was supported by mild to moderate histopathological changes in the kidneys of rats that received aflatoxin B1.
There was a significant increase in the relative spleen weight in the rats after aflatoxin B1 administration, which might be due to passive venous congestion as a result of acute toxicity of aflatoxin B1. This was supported by gross and histopathological changes observed in the present study viz., splenomegaly, massive congestion and extensive haemorrhagic areas.
Histopathological lesions observed in AFB1 treated rats at 14, 21 and 28 day of experimental study are in accordance with the research findings of Barton et al. (2000) and Preetha et al. (2006) who also found similar lesions upon AFB1 administration to rats in AFB1 toxicity studies.
Conversion of AFB1 to its highly reactive 8,9-epoxide by the action of the mixed function mono-oxygenase enzyme systems (cytochrome P450-dependent) in the tissues (in particular, the liver) of the affected animal might lead to hepatic damage observed in AFB1 toxicity. This epoxide is highly reactive and can form derivatives with several cellular macromolecules, including DNA, RNA and protein (Mclean and Dutton, 1995).
The histological examination of AFB1+AGE treated liver on day 14, 21 and 28 showed very mild lesions, also supported grossly by normal size, shape and color of liver and other organs. Our results are in line with the findings of Tadi et al. (1991) who also found mild lesions in AFB1+garlic extract treated liver compared to severe lesions in AFB1 treated liver. Srikanth et al. (2013) reported mild lesions in Acetaminophen +AGE treated liver compared to severe lesions noticed in acetaminophen treated liver suggesting that AGE treatment protected the liver damage induced by AFB1.
The hepatoprotective effect could be attributed to antioxidant and anti-lipid peroxidative effects of garlic. Garlic contains several organosulfur compounds such as allicin, diallyl sulfide, and diallyl disulfide which are valuable precursors for glutathione biosynthesis (Tadi and Lau, 1990). It is possible that garlic prevented glutathione depletion induced by AFB1 by up regulating its biosynthesis in the liver.
The histopathological changes observed in the kidney of AFB1 treated Group on day 14, 21 and 28 of the experiment could be attributed to inflammatory response to AFB1 induced injury. The histopathological changes on day 14, 21 and 28 of the experiment were milder compared to lesions noticed in AFB1 control group, could be due to antioxidant and anti-lipid peroxidative effects of AGE (Takefumi et al., 2006).
The histopathological changes observed in the spleen of AFB1 treated Group on day 14, 21 and 28 of the experiment could be due to inflammatory response to AFB1 induced injury. The severe lymphocytolytic activity observed in the present study could be attributed to immunosuppressant activity of AFB1. The milder histopathological changes observed in spleen of AFB1+AGE treated group on day 14, 21 and 28 of the experiment could be attributed to anti-inflammatory effects of AGE, the decreased lymphocytolytic activity could be due to immunoprotective effect of AGE (Wang et al., 1998).
Conclusion
It was concluded that hepatic damage can be experimentally induced in rats by administration of AFB1 at the dose rate of 4 mg/kg B.W by intraperitoneal route. Hepatic damage observed in AFB1 toxicity could be attributed to the conversion of AFB1 to highly reactive 8, 9-epoxide by the action of mixed function mono-oxygenase enzyme systems (cytochrome P450-dependent) in liver. Administration of AGE orally through orogastric tube, for 28 days had significant hepatoprotective effect in AFB1 induced liver damage in rats. Hepatoprotective effect could be attributed to antioxidant, detoxifying enzyme systems and anti-lipid peroxidative effects of AGE.
Acknowledgement
Authors of the manuscript thank and acknowledge their respective Universities/Institutes.
Conflicts of Interest
The authors declare that no conflict of interest.
Authors’ Contribution
All authors contributed equally.
References






