Advances in Animal and Veterinary Sciences
Research Article
Histopathological Changes in Grossly Normal Caprine Mesenteric Lymph Nodes
Shailesh Kumar Patel1*, Katherukamem Rajukumar2, Rakesh Kumar1, Balena Venkata Rami Reddy1, Rajendra Singh1, Palanivelu Munuswamy1, Jigyasa Rana3
1Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly-243122, Uttar Pradesh, India; 2ICAR-National Institute of High Security Animal Diseases, Bhopal-462022, Madhya Pradesh, India; 3Department of Veterinary Anatomy, C.V.A.Sc, G. B. P. U. A&T, Pantnagar-263145, Uttarakhand, India.
Abstract | Mesenteric lymph nodes (MLN) are good indicators of intestinal health of animals. Lesions of MLN are commonly associated with infectious and malignant diseases. Unsuspected altered MLN are frequently encountered during necropsy. The aim of this study was to document microscopic findings in grossly normal MLN collected from apparently healthy goats. Mesenteric lymph node samples of 40 apparently normal goats were collected from goat slaughter house in Bareilly. Based on microscopic examination, the lesions were classified into four categories, namely inflammatory changes, reactive hyperplasia, lymphoid depletion and necrosis. Microscopic examination showed that ten out of the 40 (25%) MLN had inflammatory changes in cortex as well as medulla, six (15%) had cortical and para-cortical lymphoid hyperplasia, three (7.5%) showed lymphoid depletion in the cortex and five (12.5%) lymph nodes had necrotic lesions in the cortical area. Sixteen out of the 40 (40%) MLN did not have any significant histological changes. The results of the study showed that 60% of grossly normal MLN had significant microscopic lesions indicating widespread unapparent infections in goats.
Keywords | Mesenteric lymph nodes, Goats, Histopathology, Inflammatory changes, Reactive hyperplasia
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 11, 2015; Revised | August 10, 2015; Accepted | August 11, 2015; Published | December 13, 2015
*Correspondence | Shailesh Kumar Patel, Division of Pathology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareily, Uttar Pradesh, India; Email: shaileshpatel624@gmail.com
Citation | Patel SK, Rajukumar K, Kumar R, Reddy BVR, Singh R, Munuswamy P, Rana J (2016). Histopathological changes in grossly normal caprine mesenteric lymph nodes. Adv. Anim. Vet. Sci. 4(1): 53-56.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.1.53.56
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Patel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The total goat population in India is around 120 million which provide subsistence to landless and marginal farmers in the form of high quality protein, meat, milk, skin and fiber (Mir et al., 2013). Goats suffer from many disease conditions including bacterial, viral, fungal, parasitic and toxic agents (Merck Veterinary Manual, 2008). Lymph nodes are the most specialized secondary lymphoid organ. They are encapsulated, bean-shaped structures that include networks of stromal cells packed with lymphocytes, macrophages, and dendritic cells (Surinder, 2012). Connected to both blood vessels and lymphatic vessels, lymph nodes are the first organized lymphoid structure to encounter antigens that enter the tissue spaces. The lymph node provides ideal microenvironment for encounter between antigen and lymphocytes and productive, organized cellular and humeral immune responses (Fu and Chaplin, 1999).
Structurally, a lymph node can be divided into three roughly concentric regions: cortex, paracortex, and medulla, each of which supports a distinct microenvironment. The outermost layer, the cortex, contains lymphocytes (mostly B cells), macrophages, and follicular dendritic cells arranged in follicles (Jubb et al., 1985). Beneath the cortex is the paracortex, which is populated largely by T lymphocytes and also contains dendritic cells that migrated from tissues to the node. The medulla is the innermost layer, and the site where lymphocytes exit the lymph node through the outgoing (efferent) lymphatics. It is more sparsely populated with lymphoid lineage cells, which include plasma cells that are actively secreting antibody molecules (Jubb et al., 1985). Antigen travels from infected tissue to the cortex of the lymph node via the incoming (afferent) lymphatic vessels, which pierce the capsule of a lymph node at numerous sites and empty lymph into the subcapsular sinus (Jubb et al., 1985; Owen et al., 2013).
Lymph nodes are one of the important indicators which reflect gross and microscopic changes in the organs which they drain as it helps in tissue fluid filtration and are a site for lymphocyte production. The present study was undertaken to investigate the extent of histopathological changes in normal looking mesenteric lymph nodes in goats.
MATERIAL AND METHODS
Collection of Sample
A total of 40 samples of MLN were collected from goat slaughter house, Bareilly. All samples were collected from apparently healthy goats in which 27 were male and 13 were female. Age group was ranging from 1 to 3 years.
Histopathology
Tissue pieces of approximately 5 mm thickness were fixed in 10% neutral buffer formalin and processed for histopathology as per the procedures given by Luna et al. (1968). The tissues after proper fixation trimmed to 1.5 to 2 mm thickness. After trimming these tissues were washed in running tap water, in ascending grades of ethyl alcohol for dehydration, in benzene solution for clearing and then embedded in paraffin wax. Sections of 3-5 micron thickness were then cut for staining with hematoxylin and eosin (H&E).
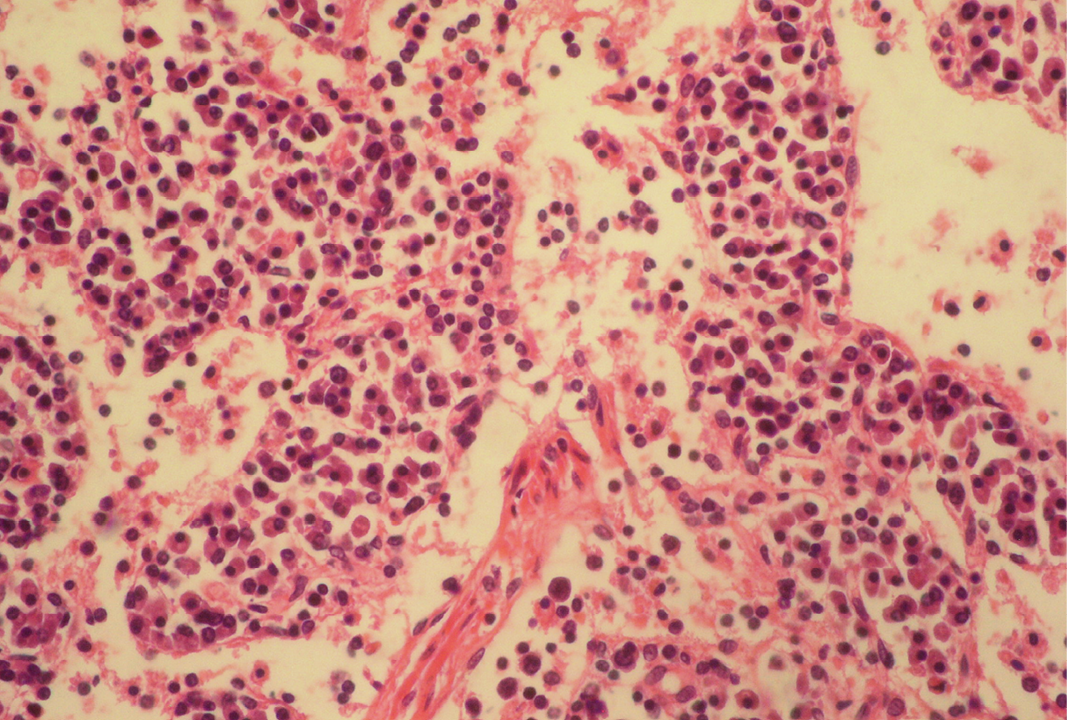
Fibrin threads are found throughout the medulla with lymphoid depletion; H&E x400
RESULTS AND DISCUSSION
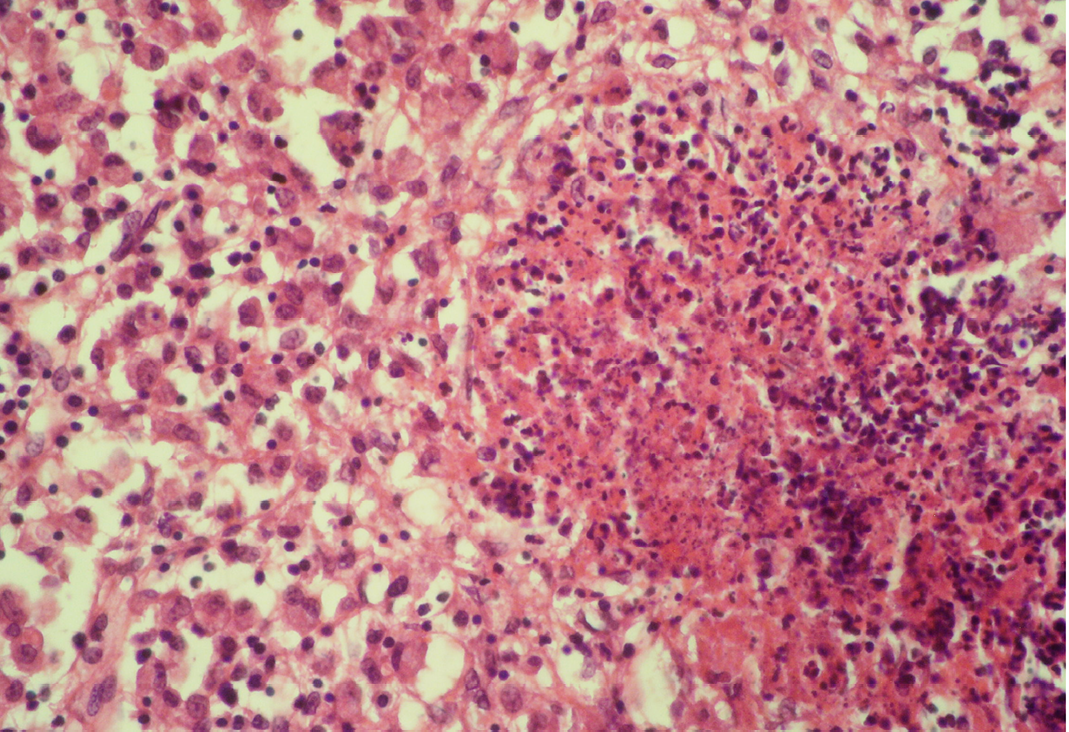
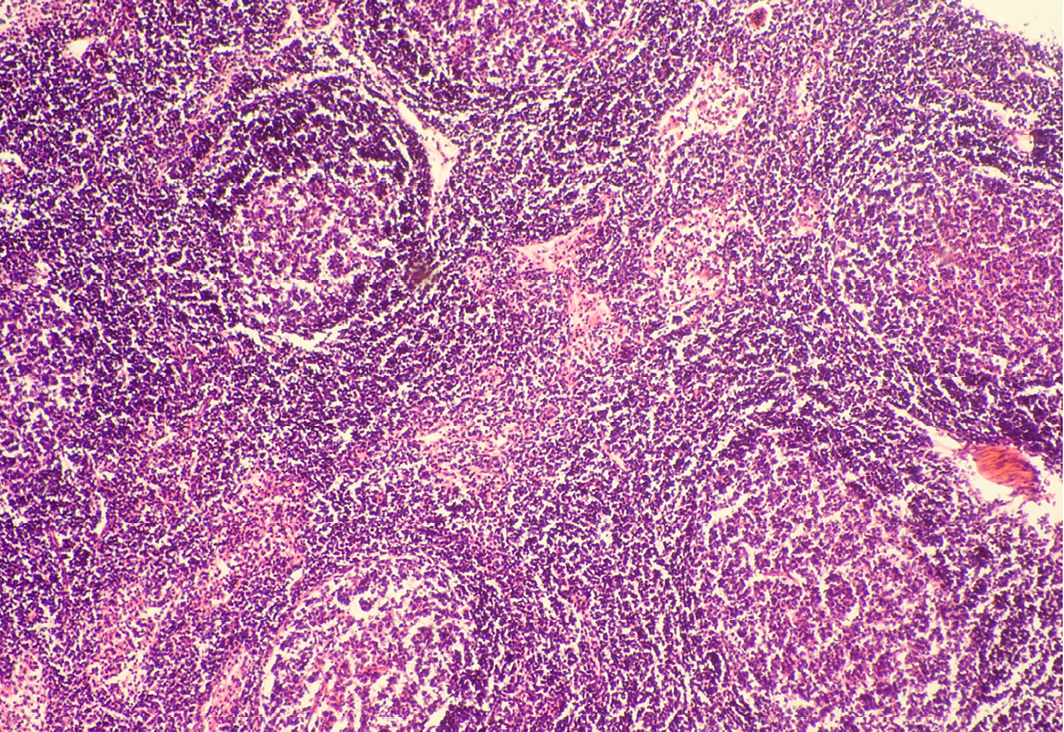
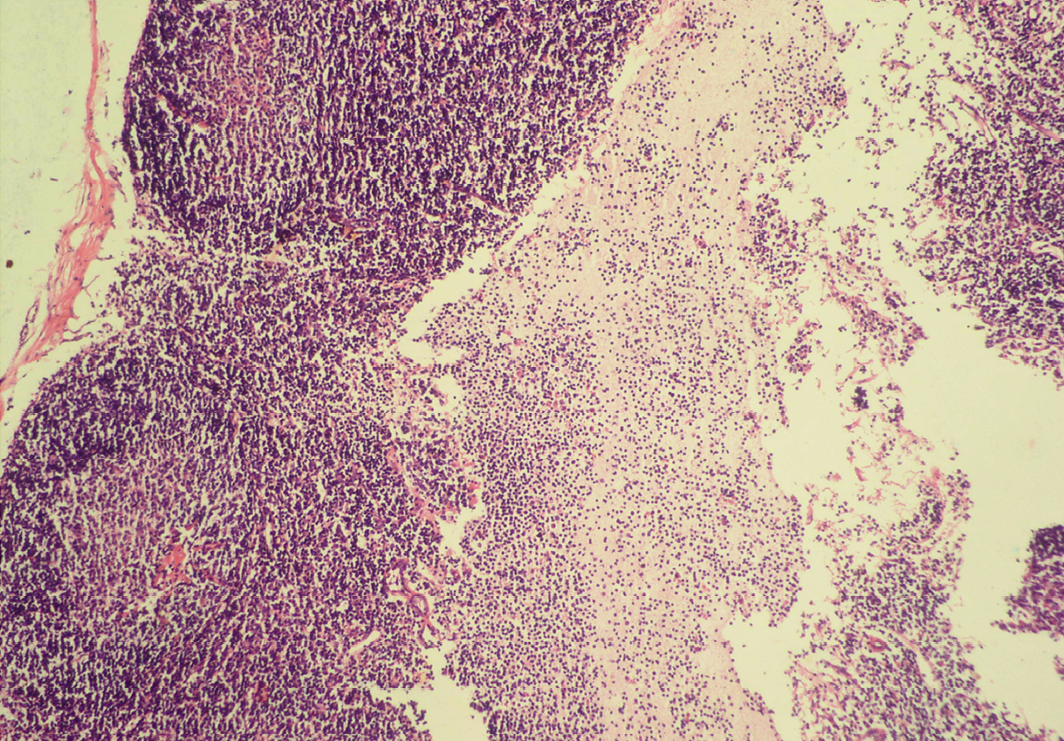
Seventeen out of 40 necropsied goats showed slightly or moderately enlarged mesenteric lymph nodes, while no obvious lesions were observed in MLN of other goats. Among the representative 40 samples studied 6 lymph nodes were hyperplastic showing increased number of follicles in cortical area (Figure 3), 10 lymph nodes showed inflammatory changes like infiltration of mononuclear cells along with fibrin threads. 5 lymph nodes showed necrotic changes in cortical area (Figure 1) which was surrounded by macrophages (histiocytes) and also showing characteristic karyolytic and karyorrhactic changes (Figure 2). Three lymph nodes showed depletion of follicles in cortex and a sheet of inflammatory cells present over homogenous meshwork of fibrin strands (Figure 4 and 5). 16 lymph nodes were found apparently normal. Among the 40 lymph nodes 25% of lymph nodes were inflammatory, 15% were hyperplastic, 12.5% were necrotic and 7.5% showed depletion whereas 40% lymph nodes were apparently normal.
The area is surrounded by macrophages (histiocytes); H&E x400
In cortex, number of follicles is increased and also showing increased number of lymphocytes; H&E x100
Lymph node hyperplasia can involve both the T-cell-rich paracortex and B-cell-rich follicles and can be indicative of a cell-mediated or humoral response, respectively (Elmore, 2006a). Mesenteric lymph nodes may show a wide variation in degree of reactive hyperplasia between animals due to stimulation by antigens in the gastrointestinal tract (Elmore, 2006b). Lymphoid hyperplasia is generally a reactive or immune response and is not considered to be a preneoplastic lesion in the lymph node (Elmore, 2006b; Ward, 1990).
Sheet of inflammatory cells are present over homogenous meshwork of fibrin strands; H&E x100
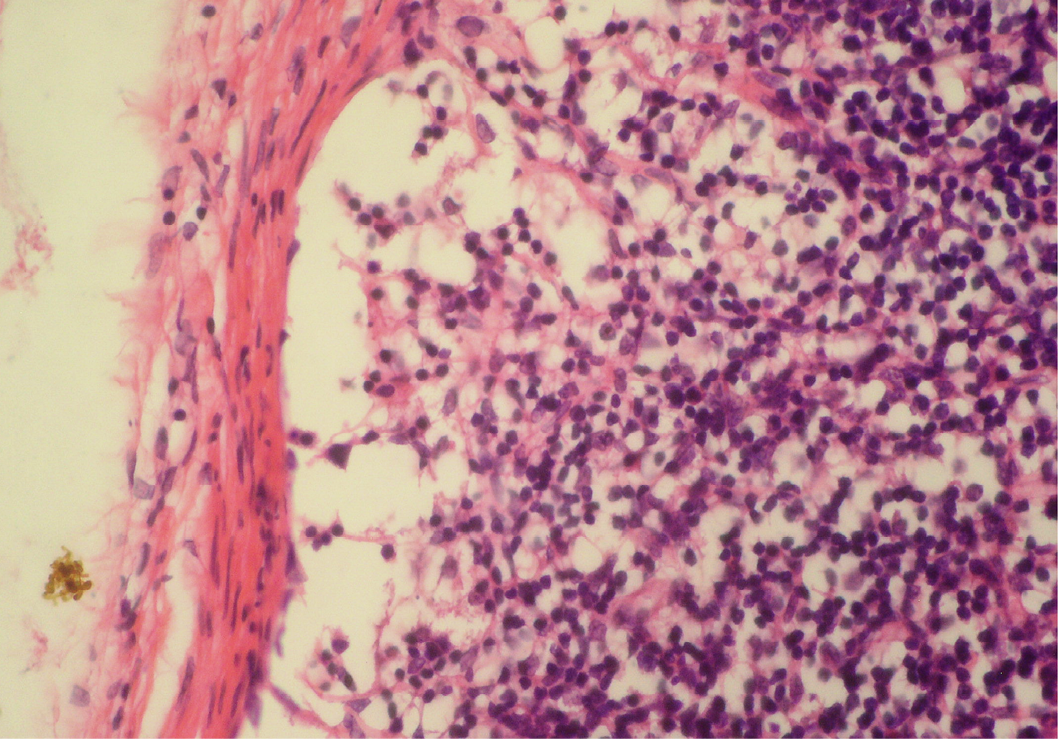
Figure 5: Depletion of follicles in cortex
Thinning of cortex and widened subcapsular space, fibrin threads are also visible; H&E x400
Lymphoid necrosis may either be diffuse, focal or multifocal within a lymph node and there can be differences in the presence and severity of lymphoid necrosis between lymph nodes in the same animal, depending on the effectiveness of the immune response. Lymphocyte necrosis is characterized by karyorrhexis, karyolysis or chromatin clumping and in more severe cases abundant eosinophilic cellular debris may be found. Necrosis is frequently accompanied by presence of inflammatory cells including neutrophils and phagocytic macrophages with intracytoplasmic cellular debris (Elmore, 2006a).
Inflammation of mesenteric lymph node can vary depending on the causal agent like bacteria, viruses, fungi, parasites, foreign bodies etc. and the response can vary from acute to granulomatous. Inflammatory cells can also be present within a lymph node in response to necrosis. In acute lymphadenitis, neutrophils can be found within the sinuses and medullary cords whereas in chronic lymphadenitis plasma cells and fibrin threads can be found. Lymphoid depletion in lymph nodes may suggestive of BVDV (Nelson et al., 2008; Krametter-Froetscher et al., 2010). In PPR infection variable degrees of lymphoid cell depletion may be found in mesenteric lymph node (Aruni et al., 1998; Ahmed et al., 2005).
CONCLUSION
It was concluded from the present study that 60% of grossly normal MLN had significant microscopic lesions indicating that incidental abnormal findings of MLN are common, reflecting more widespread unapparent infections in goats which were not evident clinically but seen only at the time of post-mortem.
ACKNOWLEDGEMENT
The authors are thankful to the Director and Joint Director (Academic), ICAR-Indian Veterinary Research Institute (ICAR-IVRI), Izatnagar, Uttar Pradesh, and Head, Division of Pathology, ICAR-IVRI for providing all facilities to carry out histopathological work.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR’S CONTRIBUTION
All the authors contributed equally.
REFERENCES