Advances in Animal and Veterinary Sciences
Case Report
Advances in Animal and Veterinary Sciences 2 (5): 302 – 304Rapid Detection of Fusobacterium necrophorum as a Main Causative Agent of Foot Rot in Small Ruminants by Polymerase Chain Reaction Assay
Shivasharanappa Nayakwadi2*, Vivek Kumar Gupta1, Nitika Sharma2, Anil Kumar Mishra2, Vikas Kumar Singh4, Kumaresan Gururaj1, Dinesh Kumar Sharma2, Rajveer Singh Pawaiya1, Ashok Kumar1, Souvik Paul2, Naveen Kumar3, Shoor Veer Singh1
- Division of Animal Health, Central Institute for Research on Goats, Makhdoom, P.O. Farah, Mathura (U.P)
- Division of Animal Health, Central Institute for Research on Goats, Makhdoom, Post Office. Farah, Mathura (U.P)
- Division of Animal Health, Central Institute for Research on Goats, Makhdoom, P.O. Farah, Mathura (U.P)
- Veterinary Immunology section, Indian Veterinary Research Institue, Izatangar
*Corresponding author: drshivasharan@gmail.com
ARTICLE CITATION:
Shivasharanappa N , Gupta VK, Sharma N, Mishra AK, Singh VK, Pawaiya RVS, Gururaj K, Sharma DK, Kumar A, Paul S, Kumar N, Singh SV (2014). Rapid detection of Fusobacterium necrophorum as a main causative agent of foot rot in small ruminants by polymerase chain reaction assay. Adv. Anim. Vet. Sci. 2 (5): 302 – 304.
Received: 2014–06–03, Revised: 2014–06–11, Accepted: 2014–06–12
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.5.302.304
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The study was aimed at detection of Fusobacterium necrophorum and Dichelobacter nodosus directly from field samples of goats and sheep with the aid of PCR assay targeting lktA and 16S rRNA genes, respectively. A total of 24 swab DNA samples from foot lesions suspected for foot rot tested for lktA and 16S rRNA, 19 (79%, Goats– 17 and Sheep–02) were found positive for lktA gene of F. necrophorum but negative for 16S rRNA gene of D. nodosus. This study shows that F. necrophorum alone could cause foot rot in absence of D. nodosus in goats.
INTRODUCTION
Several studies suggest that over 80% of sheep flocks contain lame animals (Wassink et al., 2003a and Wassink et al., 2003b). Foot rot is a highly contagious bacterial disease affecting feet of small ruminants caused by the Fusobacterium necrophorum and Dichelobacter nodosus (Beveridge, 1941; Egerton et al., 1969) which is characterized by interdigital tissue necrosis leading to lameness which can result in serious economic losses to small ruminant production. F. necrophorum causes most of the inflammation and tissue damage, whereas D. nodosus produces enzymes responsible for invasion and separation of the horn (Hodgkinson, 2010). The distinctive lesions and bacterial culture can help to diagnose the disease. The PCR is one of the sensitive assays in detecting presence of these agents from the swabs collected from interdigital necrotic lesions in cattle (Bennett et al., 2009). There are few studies about the role of F. necrophorum involvement in the foot rot in goats. Culture and isolation of these causative agents in the laboratory are cumbersome and require specical media and time duration of 3–4 weeks. Therefore, we used a PCR assay targeting lktA gene of Fusobacterium necrophorum and 16S rRNA gene of Dichelobacter nodosus from goats and sheep with foot lesions suspecting of foot rot.
A total of 24 sterile cotton swabs (19 goats and 05 sheep) of foot rot exudates from animals showing lameness and interdigital necrotic/suppurative lesions were collected and were frozen at –80oC until processed. DNA was isolated using Wizard® Genomic DNA Purification Kit (Promega) and subjected to PCR assay for the detection of F. necrophorum and D. nodosus using primers for amplification of leukotoxin structural gene (lktA) of F. necrophorum and 16S rRNA of D. nodosus. Primers used in the study are described in Table 1. The PCR thermal profile consisted of an initial denaturation step at 94oC for 2 min, followed by 35 cycles of 95oC for 30 s, 59oC for 30 s and 68oC for 30 s. A final extension of 5 min at 72oC was performed. Amplification was performed in a 25 uL reaction mixture containing 1uL of DNA, 0.30 uM of each primer, 12.5 uL of FideliTaq PCR Master Mix (2X) (Affimatrix®), 0.4 mM of dNTPs , 3 mM of MgCl and 0.5U of FideliTaq DNA Polymerase (Affimatrix®). Amplification was carried out in a Gradient thermocycler (Eppendorf), and the thermal profile consisted of a denaturation cycle at 95oC for 2 min, followed by 35 cycles of 95oC for 30 s, 60oC for 30 s and 68oC for 30 s, with a final extension step at 72oC for 5 min. PCR products from both the lktA and 16S rRNA genes were separated of ethidium bromide, and visualized under UV light using a transilluminator.
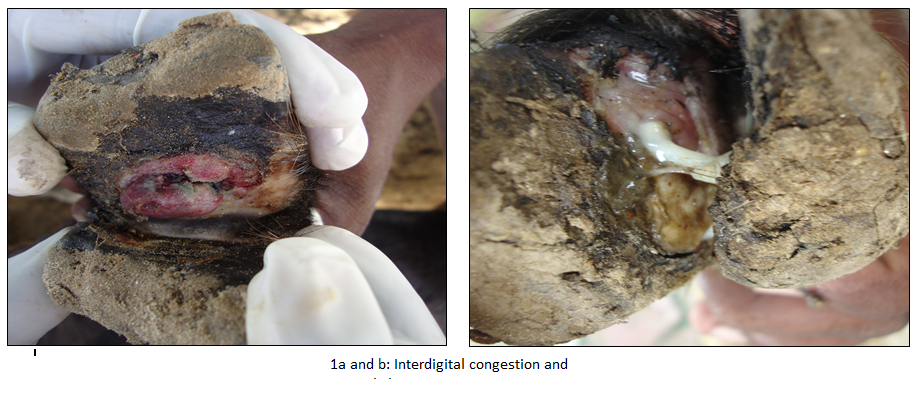
A total of 24 animals (19 Goats and 05 sheep) have shown clinical signs such as lameness and recumbence (5 goats). Clinical examination of feet revealed interdigital necrosis, bleeding, pus formation and sloughing of tissues (Figure 1a and b). Out of 24 DNA swab samples of goats and sheep tested for lktA and 16S rRNA in PCR, 19 (79%, Goats–17 and Sheep–02) revealed amplification of 402bp fragment, which was correspondingto lktA gene of F. necrophorum. Surprisingly none of the samples revealed amplification of 16S rRNA gene of D. nodosus (Figure 2).This study describes the detection of F. necrophorum from swab samples of exudates from foot rot in goats and sheep by PCR assay targeting lktA gene without going for isolation and culture. Interdigital necrosis and suppurative lesions in goats and sheep examined in our study may be due to different conditions such as Interdigital dermatitis (ID), benign footrot or very early virulent footrot (Winter, 2004). F. necrophorum is an opportunistic pathogen and has been associated with many disease conditions, such as footrot, hepatic abscess and necrotic laryngitis in animals (Nagaraja et al., 2005). It possess a leukotoxin gene (lktA) and express a leukotoxin which is considered to be the main virulence factor (Coyle–Dennis and Lauerman, 1979) and is unique to F. necrophorum, as it is not present in other Fusobacterium species (Oelke et al., 2005; Bennett et al., 2009). F. necrophorum may cause inflammation of the interdigital skin (i.e. interdigital dermatitis) and produce a number of toxins which cause necrosis of the superficial layer of the interdigital skin and enable the establishment of other bacteria, including D. nodosus (Beveridge, 1941). The diagnosis of footrot is a tedious and time–consuming process, complicated by the fastidious growth requirements and slow growing nature of these organisms (Wani and Samanta, 2006). Therefore, PCR assay was employed for detecting foot rot condition in animals by targeting lktA (Bennett et al., 2009), 16sRNA (Bennett et al., 2009), and IntA gene (Cheetham et al., 2006) can be used as rapid, sensitive and specific test for diagnosis of foot rot in animals from swab samples of exudates. When employed, PCR assay vastly improved the accuracy of identification and grouping of D. nodosus from footrot lesions (John et al., 1999).
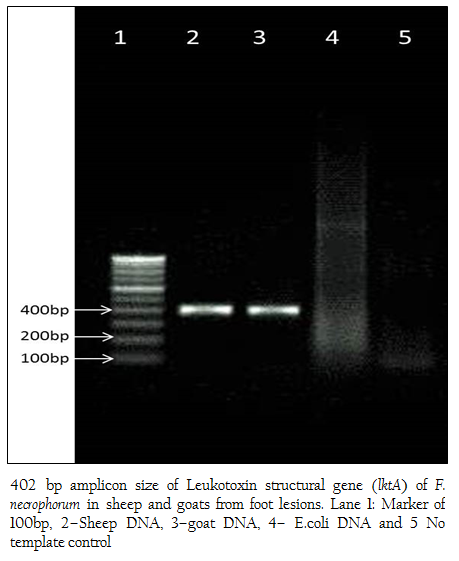
Figure 2: 402 bp amplicon size of Leukotoxin structural gene (lktA) of F. necrophorum in sheep and goats from foot lesions. Lane 1: Marker of 100bp, 2–Sheep DNA, 3–goat DNA, 4– E.coli DNA and 5 No template control
Our findings suggested that F. necrophorum is the main causative agent of foot rot in goats as in 79% of cases no D. nodosus detected. All the 79% cases in goats were in advance stages and were showing suppurative and necrotic lesions in feet. However, the presence of D. nodosus in lesions cannot be ruled out without bacterial isolation. It is interesting to note that, on the contrary to the available literature, D. nodosus seems to be a major player in foot rot in goats. However, further studies are required to establish the fact. Therefore, PCR based diagnosis can be efficiently used in the detection of F. necrophorum which are otherwise difficult to culture in common laboratory conditions. The presence of F. necrophorum has implications in the management of flock health in semi–intensive rearing system in India. Hence PCR assay can be employed to detect the presence of mixed infection by these bacteria in feet lesions of small ruminants. The presence of lktA gene indicated the presence of virulent strain of F necrophorum which was responsible for clinical foot rot in goats and sheep. Further studies envisaged on large number of samples collected from different areas are required to ascertain the fact that the F. necrophorum is the main causative agent of foot rot in goats.
REFERENCES
Bennett G, Hickford J, Sedcole R, Zhou H (2009). Dichelobacter nodosus, Fusobacterium necrophorum and the epidemiology of footrot. Anaerobe. 15(4): 173–176.
http://dx.doi.org/10.1016/j.anaerobe.2009.02.003
http://dx.doi.org/10.1016/j.anaerobe.2009.02.002
PMid:19239925
Bennett G, Hickford J, Zhou H, Laporte J, Gibbs J (2009). Detection of Fusobacterium necrophorum and Dichelobacter nodosus in lame cattle on dairy farms in New Zealand. Research in Veterinary Science 87(3): 413–415.
http://dx.doi.org/10.1016/j.rvsc.2009.04.001
PMid:19409584
Beveridge WIB (1941). Foot–rot in sheep: a transmissible disease due to infection with Fusiformis nodosus. Bulletin No. 140. Council for Scientific and Industrial Research, Melbourne, Australia.
PMid:17734549
Cheetham BF, Tanjung LR, Sutherland M, Druitt J, Green G, McFarlane J, Katz ME (2006). Improved diagnosis of virulent ovine footrot using the intA gene. Vet. Microbiol. 116(1): 166–174.
http://dx.doi.org/10.1016/j.vetmic.2006.04.018
PMid:16716540
Coyle–Dennis JE, Lauerman LH (1979). Correlations between leukocidin production and virulence of two isolates of Fusobacterium necrophorum. Am. J. Vet. Res. 40: 274–276.
PMid:464365
Egerton JR, Roberts DS, Parsonson IM (1969). The aetiology and pathogenesis of ovine footrot. J. of Comp. Pathol. 79: 207–216.
http://dx.doi.org/10.1016/0021-9975(69)90007-3
Hodgkinson O (2010). The importance of feet examination in sheep health management. Small. Rum. Res. 92(1): 67–71.
http://dx.doi.org/10.1016/j.smallrumres.2010.04.007
John G, Smith R, Abrahm KJ, Ellis RP (1999). Identification and grouping of Dichelobacter nodosus, using PCR and sequence analysis. Mol. and Cel. Prob. 13: 61–65.
http://dx.doi.org/10.1006/mcpr.1998.0210
PMid:10024434
Nagaraja TG, Narayanan SK, Stewart GC, Chengappa MM (2005). Fusobacterium necrophorum infections in animals; pathogenesis and pathogenic mechanisms. Anaerobe. 11: 239–246.
http://dx.doi.org/10.1016/j.anaerobe.2005.01.007
PMid:16701574
Oelke AM, Nagaraja TG, Wilkerson MJ, Stewart GC (2005). The leukotoxin operon of Fusobacterium necrophorum is not present in other species of Fusobacterium. Anaerobe. 11: 123–129
http://dx.doi.org/10.1016/j.anaerobe.2004.10.003
PMid:16701542
Wani SA, Samanta I (2006). Current understanding of the aetiology and laboratory diagnosis of footrot. The Vet. J. 171(3): 421–428.
http://dx.doi.org/10.1016/j.tvjl.2005.02.017
PMid:16624707
Wassink GJ, Grogono–Thomas R, Moore LJ, Green LE (2003a). Risk factors associated with the prevalence of footrot in sheep from 1999 to 2000. Vet. Rec. 152: 351–358.
http://dx.doi.org/10.1136/vr.152.12.351
PMid:12678258
Wassink GJ, Green LE, Grogono–Thomas R, Moore LJ (2003b). Footrot in sheep. Vet. Rec. 153: 572.
PMid:14627244