Advances in Animal and Veterinary Sciences
Research Article
Antifungal Activity of Thyme and Carvacrol Nanoemulsions Against Aspergillus fumigatus Isolated from Different Types of Cheese
Eman M. Shaker1*, Marwa M. Farghaly2
1Department of Food Hygiene, Faculty of Veterinary Medicine, Sohag University, Egypt; 2Division of Microbiology, Botany Department, Faculty of Science, Sohag University, Egypt.
Abstract | The control of fungal spoilage is a major concern for industrials and scientists that are looking for efficient solutions to prevent and/or limit the fungal spoilage in dairy products. Therefore, the aim of this study is controlling Aspergillus fumigatus isolated from cheese by safe, non-allergic and natural food preservative nano-emulsions. Two hundred cheese samples of four different types (50 each) were studied for the presence of A. fumigatus. The isolated strains were examined for their ability to growth under the effect of two natural nano-emulsion as thyme (TN) and carvacrol (CN) nanoemulsions using well diffusion method (WDM). The possible changes in the fungus structure were described by scanning electron microscope (SEM). The nanoemulsions were prepared and characterized by transmission electron microscope (TEM). The incidence of A. fumigatus in different types of cheese was detected with percentage of 24, 6 and 12 % in Kareish, Damietta and Processed cheeses, respectively and could not be detected in Cheddar cheese. Results show a significant inhibitory effect of CN than TN on A. fumigatus growth with distinctive destruction in its ultrastructure. Applications of CN in the dairy industry will be a market trend to improve cheese quality by their antifungal properties and enhancement their shelf life.
Keywords | Kareish cheese, Damietta cheese, Processed cheese, Thyme nanoemulsion, Carvacrol nanoemulsion
Received | August 12, 2021; Accepted | August 18, 2021; Published | September 25, 2021
*Correspondence | Eman M Shaker, Department of Food Hygiene, Faculty of Veterinary Medicine, Sohag University, Egypt; Email: milk_121970@yahoo.com
Citation | Shaker EM, Farghaly MM (2021). Antifungal activity of thyme and carvacrol nanoemulsions against aspergillus fumigatus isolated from different types of cheese. Adv. Anim. Vet. Sci. 9(11): 1945-1950.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1945.1950
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shaker and Farghaly. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Nowadays, there is an interest in providing healthy foods with a prolonged shelf life for human being. Food spoilage fungi considered a big problem in the dairy industry, especially cheese manufacturing, this adaptation capacity may be due to the ability of fungi to utilize many substrates including lactose, organic acids, proteins, and milk fat that are present in milk and its products. Moreover, these fungi are acidotolerant, xerotolerant, and/or psychrotolerant, and to some extent can tolerate chemical preservatives, which are sometimes added to increase the product shelf-life (Prado et al., 2015).
Aspergillus species, particularly A. fumigatus spores, are spreading within the air and pose a high risk of exposure for the dairy products along the production and ripening steps, or comes from the original milk as it causes mastitis in animal and can withdraw with milk. This fungus not only causes spoilage to dairy food during storage but also is able to produce thermogenic mycotoxins with genotoxic, cytotoxic, immunosuppressive and apoptotic effects for human health by eating moldy food (Hof, 2016; Blachowicz et al., 2020).
There is an increased trend for demand healthy food products, as cheese, without synthetic additives and environmentally friendly food production technologies (Burt, 2004). One of these strategies is adding plant essential oils and their constituents as antimicrobial additives, which show antimicrobial activity against different toxicogenic and infective microorganisms (Nguefack et al., 2009). Constituents of essential oils like thyme and carvacrol show antifungal activity against a wide range of fungi. They could be used as a safer alternative to chemical preservatives in the food industry and give the same effect (Tajkarimi et al., 2010; Pérez-Alfonso et al., 2012).
The antifungal activity of thyme essential oil on A. fumigatus is associated with the damage to the cell wall and cytoplasmic contents. Furthermore, penetration of plasma membrane by its lipophilic properties which allows it to causing polysaccharide accumulation under drought stress conditions and leading to plasmalemma breakage in fungal cells (Ferreira et al., 2013). Carvacrol essential oil has been shown to increase membrane fluidity and cause leakage of protons and potassium ions, resulting in a collapse of membrane potential and inhibition of adenosine triphosphate (ATP) synthesis and disrupt the fungal cell metabolism (Suntres et al., 2015).
To increase the antifungul effect of the essential oils in the food, the doses used might exceed organoleptically acceptable levels. So, the involvement of nanotechnology in the food trade has led to produce nanemulsions with different physical properties (He and Hwang, 2016) such as increasing the dispersibility of essential oils in aqueous formulations, protect it from degradation, increase chemical stability during storage, minimize organoleptic changes by masking of an unwanted flavor or taste of the core material; the possibility for applications of oil encapsulation in the food industry, and improve their antimicrobial action (Weiss et al., 2009).
So, the aim of this study to detect the incidence of A. fumigatus in four different types of cheese and examine the possible use of nanoemulsions of thyme and carvacrol essential oils as a natural antifungal through inhibition of fungal growth and deformation of fungal ultrastructure.
Materials and methods
Collection of samples and mycological examination
Different types of cheese, including 200 samples of Kareish, Damieta, Cheddar and Processed cheese (50 of each type), were purchased from different markets in Sohag city for detection of A. fumigatus. All samples were prepared according to (APHA, 2003) for fungal examination. The prepared samples were cultured on potato dextrose agar (PDA) with antibiotics at 25 ˚C for 5 to 7 days (ELShibiny el al., 2013). Identification of A. fumigatus was done by morphological structure, microscopically, then by SEM in Assiut University Unit of Electron Microscope.
Synthesis of TN and CN
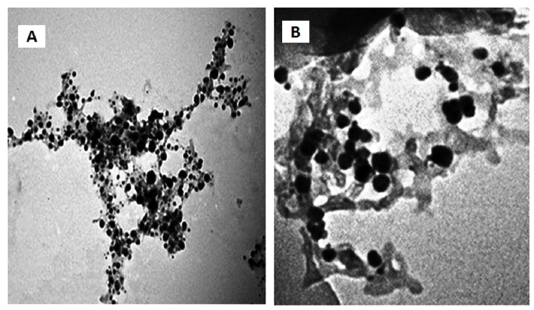
2v/v% Tween 80 at room temperature was dissolved in distillated water. The mixture with a magnetic type stirrer was shaken for 10 minutes to form a homogeneous solution. Then, added essential oils slowly and mixed for 15 minutes with a direct driven stirrer (Daihan Scientific Homogenizer HG-150). The resulting crude emulsion was sonicated using a 25 kHz ultrasonic homogenizer (USH650, max power: 650 watt) (Ghosh et al., 2013). The nanoemulsions were prepared in Nanotechnology Research Unit, AHRI, ARC, Egypt. The size of both nanoemulsions was measured by TEM Model JEOL-JEM-100CX II in the Electron Microscopy Unit, Assiut University, Egypt.
Antifungal effect of TN and CN
A. fumigatus which isolated from different types of chesse were grown in potato dextrose liquid medium at 25ºC for 6 days, and then 1 ×106 CFU/mL were plated on fresh PDA. By using well diffusion method, 80 µl of various concentrations of previously prepared nanoemulsions (0.5, 1, 2, 3 and 4%) were transferred onto the wells, and the agar medium plates were incubated at the temperature of 25ºC for 6 days. Finally, the anti- A. fumigatus activity was determined by measuring the growth inhibition zones, while their effect on fungal ultrastructure was detected by SEM (Ksouri et al., 2017).
Statistical analysis
The effect of TN and CN was described by using SPSS 16, USA for mean and standard error. Significant difference used at least at p< 0.05.
Results
Incidence of A. fumigatus
The incidence of A. fumigatus from different cheeses samples was 21(10.5%) out of 200 samples. It was detected in 12 (24%) of Kareish cheese, 3 (6%) of Damietta cheese and 6 (12%) of Processed cheese. While, it cannot be detected in Cheddar cheese samples (Table 1).
Antifungal effect of TN and CN
Table 2 cleared that TN had no effect on the fungal growth at concentrations 0.5 and 1%, and their inhibitory effect was reported in concentrations 2, 3 and 4 %, with mean inhibition zones 3±0.05, 5±0.32 and 10±0.53 mm, respectively. While, the inhibitory effect of CN on A. fumigatus was detected in concentrations of 0.5, 1, 2, 3, and 4% with mean inhibition zones 10±0.78, 13±0.43, 16±0.43, 24±0.80 and 29±0.45 mm, respectively. Increased concentration of TN and CN significantly increased their antifungal effect (p value < 0.05). SEM described the damage of A. fumigatus ultrastructure due to the effect of TN and CN (Figure 2).
Table 1: Incidence of A. fumigatus in cheese samples
| Cheese type | No. of samples |
A. fumigatus |
|
| No | % | ||
| Kareish cheese | 50 | 12 | 24 |
| Damietta cheese | 50 | 3 | 6 |
| Cheddar cheese | 50 | 0 | 0 |
| Processed cheese | 50 | 6 | 12 |
| Total | 200 | 21 | 10.5 |
Table 2: Inhibitory effect of TN and CN on A. fumigatus
| Concentrations | Inhibition zone by mm |
p value |
|
| TN | CN | ||
| Mean ± SdE | Mean ±SdE | ||
| 0.5% | No zone | 10±0.78 | 0.05 |
| 1% | No zone | 13±0.43 | |
| 2% | 3±0.05 | 16±0.43 | |
| 3% | 5±0.32 | 24±0.80 | |
| 4% | 10±0.53 | 29±0.45 | |
Discussion
Incidence of A. fumigatus in different types of cheese
Molds are often added to certain varieties of cheese to provide a characteristic appearance, consistency, and flavor (Haasum and Nielsen, 1998). However, spoilage molds that contaminate cheese can produce mycotoxins, which lead to serious health hazards (Dobson, 2017). From the fast-growing and saprophytic fungus is A. fumigatus that sporulates abundantly, releasing thousands of airborne conidia which are of relatively small size (2- to 3-µm in diameter) (Croft et al., 2016). However, it can spread easily in the environments related to the pathway of cheese production either during manufacturing, ripening or storage and produce mycotoxins (Kwon-Chung and Sugui, 2013; Ashton and Dyer, 2019).
Table (1) showed the incidence of A. fumigatus in kareish cheese in a percentage of 24% , this result is higher than that mentioned by Hameed (2016), and lower than that reported by ELbagory et al. (2014), while other studies cannot detect A. fumigatus in Kareish cheese (Banjara et al., 2015; Mohamed et al., 2017; El-Shinawy et al., 2018). The presence of A. fumigatus in Kareish cheese may be due to the traditional manufacturing method applied in Sohag city, Egypt by using unpasteurized milk and curdling occurs through natural fermentation by leaving it overnight at room temperature which provide a good media for fungus growth, moreover it is sold in markets without packaging; these unhygienic control measures give a chance for contamination with A. fumigatus conidia.
The examined samples of Damietta cheese were contaminated with A. fumigatus at a percentage of 3(6%). This finding is higher than that reported by Hameed (2016) and Mohamed et al. (2017). High moisture content and low pH value of Damietta cheese could favor the growth of mold, as it needs two months for ripening before being ready to eat, where air; equipment and the tins used in packaging considering as the major source of cheese contamination.
The negative results, which obtained from Cheddar cheese goes parallel with those obtained by El-Shinawy et al., (2018). This result agrees with the excellent history of safety of Cheddar cheese production, it is a hard, ripened cheese with a long shelf life and without any surface flora, as most microbiota are killed during pasteurization process, in addition to the hygienic measured applied during manufacturing and packaging. (Khaled et al., 2015).
Regarding the results of Processed cheese, lower incidence was observed by Hameed (2016) and Mohamed et al., (2017), While relatively higher counts were recorded by ELbagory et al. (2014). Processed cheese mixture is exposed to high temperature (85ºC for 15-20 min) during cooking process which could eliminate most organisms, yet the presence of A. fumigatus indicates post processing contamination or survival of their spores (Hassan, 2010).
Impact of TN and CN
In several researches in Egypt, different nanomaterials have antimicrobial effect against many pathogenic microorganisms, so they can be used in food industry for preservation and in packaging materials (Hassanien and Shaker, 2020).
TN and CN were applied in concentrations 0.5, 1, 2, 3 and 4% with an average size 96.3 nm and 85.7 nm, respectively (Figure 1). CN represented inhibitory effect on A. fumigatus isolates at concentrations of 0.5 and 1%, unlike TN in which no zone of inhibition was detected in the same concentrations. All CN tested doses exhibited a significant effect on A. fumigatus, while only the highest concentrations of TN (2, 3, and 4%) have inhibitory effect (p < 0.05). However, the degree of inhibition is increased with the increased concentrations of both types of nanoemulsions, and CN had significant inhibitory effect in comparison with the same concentration of TN. This may be related to the smaller size of CN than TN, which increased its surface area. Nanoemulsions with small size are more effective as their surface area was increased which enhance the exposure of microorganisms to nanoemulsions (Gupta et al., 2016). These results inconsistence with Hassanien et al. (2021) who reported that, TN and CN have antifungal effect on A. fumigatus growth (Table 2).
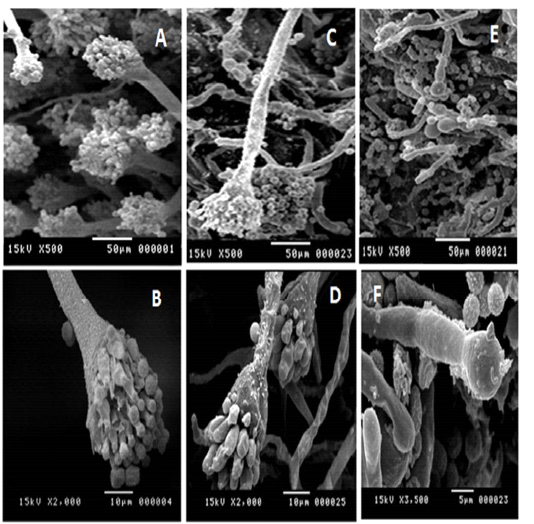
Figure 2: SEM images for A. fumigatus (A, B): normal, (C, D): effect of TN at 4%; (E, F): effect of CN at 4% on A. fumigatus
Nano-emulsions were applied in the food industry as antimicrobials, flavoring agents, and for delivery of nutraceuticals. Also, used for improving biodegradable packaging and edible coating films to increase the quality, shelf life and nutritional value of foods (Aswathanarayan and Vittal, 2019).
The antimicrobial effect of nanoemulsions related to their ability to pass through cell wall and cytoplasmic membranes into the microorganisms cells and fracture the cell structures, which increasing its permeability and causing disorders in cell metabolism, protein synthesis and ion transportation mechanism inside the cell (Langeveld et al., 2014; El-Sayed and El-Sayed, 2021).
SEM (Figure 2) explained the structure of untreated A. fumigatus and the effect of TN and CN on it. The normal structure revealed attached conidia and strong hyphae. TN at concentration of 4% causes a shrinkage and deformation in fungal hyphae and conidia. While CN, at the same concentration show fall out of fungal conidia, distorted and shrinkage of fungal hyphae. Alteration of fungal structure may affect its development and adaptation in the host (Lin et al., 2015). The alteration in the morphology of ergosterol which considered the main sterol of the cytoplasmic membrane of the fungus due to treatment with nanoemulsions will interfere with the cell wall synthesis which hinders the fungal growth (Basak and Guha, 2017). This finding revealed the antifungal properties of TN and CN which enables their use in the food industry as a food preservative or in food packaging.
Conclusion
Presence of A. fumigatus in cheese products lowers their quality and leads to their spoilage. TN and CN have antifungal effect on A. fumigatus, but CN has significant effect than TN. So, they can be used as natural food additives to improve cheese quality by investigating their antifungal properties instead of synthetic antifungal.
Acknowledgements
Nanotechnology Research Unit, Faculty of Pharmacy, Alazhar University, Assiut branch for help in preparation of nanoemulsions.
Conflict of interest
None.
Authors contribution
All authors contributed equally.
References