Advances in Animal and Veterinary Sciences
Research Article
Development of Rabbit’s Anti-Pig Serum Polyclonal Antibodies to Detect Pork Adulteration in Meat Products
Widi Nugroho1*, Karlina1, Sarah Maghfirah1, Muhamad F.A. Rasyid1, Firdauzi O. Purwanto1, Muhammad A. Lesmana2, Siska Aditya3
1Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Brawijaya, Kalisongo, Dau, Malang, East Java, Indonesia, 65151; 2Laboratory of Veterinary Surgery, Faculty of Veterinary Medicine, Universitas Brawijaya, Kalisongo, Dau, Malang, East Java, Indonesia, 65151; 3Laboratory of Veterinary Biochemistry, Faculty of Veterinary Medicine, Universitas Brawijaya, Kalisongo, Dau, Malang, East Java, Indonesia, 65151.
Abstract | This study aimed to produce rabbit’s anti-pig serum polyclonal antibodies, as part of an attempt to develop a rapid, reliable, and self-administered test for detecting pork adulteration in meat products. The pig antigen for the antibody production was obtained by the treatment of pig serum using 10% caprylic acid. Experimental immunization of rabbits using the pig antigen demonstrated that antibodies were detectable on day 56 post-initial immunization. The antibodies were capable of detecting pork juice at as low as 1% dilution. Further, the antibodies reacted specifically to pork amongst juices of pork, beef, lamb, goat, duck, and catfish meat species tested. This study provides extended knowledge for the development of a rapid, and self-administered test for detecting pork adulteration in meat products.
Keywords | Pork adulteration, Pig antigen, Caprylic acid, Rabbit’s anti-pig serum polyclonal antibodies, Agar gel immunodiffusion
Received | May 20, 2021; Accepted | August 16, 2021; Published | September 25, 2021
*Correspondence | Widi Nugroho, Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Brawijaya, Kalisongo, Dau, Malang, East Java, Indonesia, 65151; Email: widi.nugroho@ub.ac.id
Citation | Nugroho W, Karlina, Maghfirah S, Rasyid MFA, Purwanto FO, Lesmana MA, Aditya S (2021). Development of rabbit’s anti-pig serum polyclonal antibodies to detect pork adulteration in meat products. Adv. Anim. Vet. Sci. 9(11): 1919-1924.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.11.1919.1924
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Nugroho et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Pork adulterations are illegal for religious, health, or economic reasons (Ali et al., 2014; Kwon et al., 2013; Oh et al., 2004; Ramos and Canseco, 1993; Rentzos et al., 2019; Rohman et al., 2011). Pork is obviously not halal and pork consumption is religiously forbidden for two billion Muslims worldwide (Kettani, 2019). Pork adulteration can be dangerous for allergic populations. It comprises, for example, 6.8–10.2% of food allergy prevalence in Seoul, South Korea (n=755-1299), 8.8% (n=247) in Monclova, Mexico, and 0.6% (n=1042) in Västra Götaland, Sweden (Kwon et al., 2013; Oh et al., 2004; Ramos and Canseco, 1993; Rentzos et al., 2019). Yet, undeclared pork content in meat products has been detected in many countries. Studies in the world’s largest Muslim populated country, Indonesia, reported that pork adulteration occurred in 7.9% (n=33) of halal meat products in Bogor (Nida et al., 2020), and 20.0% (n=10) in Yogyakarta (Admantin and Santoso, 2013). Pork adulteration was also reported in 8.6% (n=35) of meat products in South Korea (Jimyeong Ha et al., 2017) and 25.6% (n=250) in Sichuan China (Song et al., 2019). Lack of affordable self-administered tests however, hamper consumers from being able to protect themselves from unpleasant or even detrimental effects of pork adulteration.
Efforts to develop pork detection in meat products that is easily applied by consumers have been reported. A test targeted pig-specific DNA using the LFA showed the result at as soon as 30 minutes (Magiati et al., 2019). The pig DNA fragment remains detectable in a heated meat product (200oC for 20 minutes), a seasoned pork salami, and a sausage (Ulca et al., 2013). However, due to the time length needed to complete the test, it may not be convenient when it is self-administered by consumers. Another strip test for pork detection based on DNA probing was developed and was capable of detecting pork in as soon as three minutes (Yin et al., 2020), but the sample has to be incubated at 35oC before it can be tested in the strip, a practice that may not be suitable for consumers in many daily life situations.
Immunocensor based tests to detect pork in meat products have also been reported. A test based on an anti-pig-albumin antibody was used for detecting porcine albumin in meat products but, with the electrochemical immunocensor method described, the optimum detection was obtained only after a 45-minute incubation (Lim and Ahmed, 2016). The ELISA based on monoclonal antibodies against thermal-stable fat protein of pigs, thermal-stable muscle protein of pigs or pig’s haemoglobin also have been developed. While showed excellent sensitivities and specificities, the applications of these tests were only readable after as soon as 45 minutes (Chen and Hsieh, 2000; Li et al., 2020).
A few rapid lateral flow assays (LFA) based on anti-pig-IgG polyclonal antibodies coupled with gold nanoparticles were capable of showing the test results within 2–10 minutes when were applied to both raw and cooked meat products (Depamede, 2011; Kuswandi et al., 2017; Mandli et al., 2018). Compared to other test formats, the lateral flow immunocencor test of pork adulteration is characterised as lower in cost, less time consuming, highly specific, can be done by consumers with very simple or no training, but the commercial test is currently not available (Li et al., 2020). One of the key components to manufacturing this test is the availability of anti-pig-IgG antibodies (Kuswandi et al., 2017).
To date, the production of monoclonal antibodies against pig-IgG to detect pork adulteration in meat products have not been reported (Li et al., 2020). Production of monoclonal antibodies though, when compared to polyclonal antibodies, takes longer time. It also employs more complex steps of immunisation, fusion of lymphocyte with myeloma, and culture of hybridoma cells, which need more sophisticated equipments thus can be more expensive (Wakayama et al., 2006). In fact, polyclonal antibodies against pig IgG used in an immunocencor to detect pork adulteration was capable of detecting the pork content up to 0.1%, without cross reactivity with beef (Kuswandi et al., 2017). These might underline the less importance of producing monoclonal antibodies against pig-IgG for pork detection.
The IgG and Albumin are two main components of blood plasma and the extracellular fluid of the vertebrate tissues (Brekke et al., 2010; Charles et al., 2001), making both molecules potential markers to detect pork adulteration in meat products. Immunoglobulin and Albumin can be extracted from a serum sample by simple caprylic acid (CA) treatment to precipitate other serum protein impurities (Morais and Massaldi, 2012; Shawki et al., 2017). The extraction and purification of pig immunoglobulin by CA and the capability of the filtrate to bind to specific antibodies in-vitro were reported (Bokhout et al., 1986). However, the study is lacking on the use of simple CA extract of the pig serum as a polyvalent antigen for the production of specific antibodies to detect pig species in a meat product. Provided that immunocensor based tests for pork detection have a potential for rapid and easy application by consumers, lower-cost production of the materials for fabrication of the test tool, including the production of antigen and the specific antibodies, warrant investigations.
The aim of this study, therefore, was to investigate the production, sensitivity, and specificity of an anti-pig polyclonal antibody against pig serum antigen extracted using CA, in New Zealand rabbits (Oryctolagus cuniculus). The sample used in this study was serum isolated from blood of a Landrace pig. This study provides essential knowledge for the development of rapid, reliable, self-administered, and easy to use tests for detecting pork adulteration in meat products.
Materials and Methods
Ethical consideration
The study was approved by the Animal Care and Use Committee of Universitas Brawijaya in document number 046-KEP-UB-2020. Experiment with animals was conducted in the Experimental Animal Facility, Faculty of Veterinary Medicine, Universitas Brawijaya.
Supplies of animals and meats
Three individual rabbits were used to produce anti-pig polyclonal antibodies. Four months of age, male, New Zealand White rabbits (Oryctolagus cuniculus) weighing 2.2 kg (SD ±0.1) were supplied by a local breeder in Malang, East Java, Indonesia. The serum of a Landrace pig’s blood as the source of antigen was obtained from the Kota Malang slaughterhouse. Pork, beef, lamb, goat, duck, and catfish meats for the species specificity tests were obtained from Gadang wet market in Malang.
Antigen preparation
Pig antigen was prepared by extraction of pig serum using CA with some modifications (Bokhout et al., 1986; Morais and Massaldi, 2012). The extraction was conducted in pH 5, and the final concentration of the CA was 10% (Sigma-Aldrich, St. Louis, Missouri, USA). Briefly, 20 mL pig serum was added to distilled water (OTSUKA, Surabaya, Indonesia), and CA was added drop by drop into the solution along with stirring, to a final volume of 40 mL. The mixture was vortexed for 15 minutes and subsequently centrifuged (Monota RO, Osaka, Japan) at 60,000 g for 20 minutes. The supernatant was filtered with Whatman paper No. 2 (Sigma Aldrich, St. Louis, Missouri, USA), and stored at -20oC until use.
Preparation of pig serum antigen for immunization
The protein concentration of the pig antigen was analyzed using a Nano Drop 1000™ Spectrophotometer (Thermofisher, Waltham, MA, USA). A 2 µL of the pig antigen was used, and the protein concentration was estimated at 280 nm wavelength. The pig antigen was emulsified with Freund’s complete adjuvant (G-Biosciences, St. Louis, Missouri, USA) at a final concentration of 10% (v/v) of total emulsion. The mixture was vortexed for 10 minutes, and the formation of the emulsion was tested on a water surface; small particles dispersed evenly on the surface of the water were considered proper emulsion formation (Leenaars et al., 1999).
Immunization of animals
After a week of acclimatization, each of the animals was injected with 875 µg pig serum antigen at a volume of 100 µL subcutaneously, using a one mL syringe with 26G needle. The immunization was repeated on days 7, 14, 21, and 28 after the initial immunization. Animals were bled from auricular veins using 3 mL syringes with 22G needle on day one just before the initial immunization, and on days 14, 36, and 56 after the initial immunization to analyse the development of antibodies against the pig antigen. On day 56 post-initial injection, all animals were euthanized by anesthesia followed by exsanguination, bloods were collected, and sera were extracted and stored at -20oC for further analyses (Leary et al., 2020).
Detection of antibodies against pig serum antigen
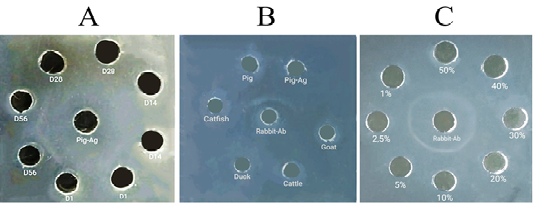
The detection of the formation of antibodies against pig antigen in immunized animals was conducted qualitatively using Agar Gel Immunodiffusion (AGID) test. Sera of experimental animals collected on day one before the initial immunization was used as the negative controls. A 1% agarose (Sigma-Aldrich, St. Louis, Missouri, USA) in 1% Phosphate Buffered Saline (PBS) (Sigma-Aldrich, St. Louis, Missouri, USA) was used in this test (da Cunha et al., 2015). A 13 mL of the liquid agarose was poured into a 20 cm diameter petri dish to form a gel. A 4 mm diameter well was punched in the center of the gel and four wells with the same diameter were punched around the central well. A 20 µL pig antigen was dropped into the central well, and 20 µL of rabbit sera collected on days 1, 14, 36, and 56 were dropped into wells designed around the central well. The gel was incubated for 48h in a humid condition, at 37oC. A white precipitation line developed between the central well contained pig antigen and the well contained serum of the immunized rabbits was deemed to indicate the formation of anti-pig antibodies.
Determination of the species specificity of the rabbit’s anti-pig polyclonal antibodies (RaPS-Ab)
Meat juices from bovine (Musculus pectoralis), goats (Musculus pectoralis), sheep (Musculus pectoralis), duck (Musculus pectoralis), and catfish (Musculus epaxialis) meat were used for this purpose. They were used in this study to represent different classes of animals from pisces, aves and mammals. Additionally, for duck and ruminant meats, the similarity in gross appearance with pork, their popularity in local context and higher prices compared to pork were considerations to include them as the subjects of the study. The meat juices were obtained by freezing the meats at -20oC followed by thawing, and juices were collected into sterile tubes, one for each species (Cybulska et al., 2020). Samples were stored at -20oC until use.
Determination of species specificity of the hyperimmune serum was determined using AGID with the same procedures described above (da Cunha et al., 2015). The central well was filled in with the hyperimmune serum and the surrounding wells were filled in with juices of the aforementioned meat species. Pork juice and the pig antigen were used as positive controls.
Determination of the sensitivity of the test
An analytical sensitivity test using AGID was performed to investigate the limit of the antigen concentration that remains detectable by the hyperimmune serum (da Cunha et al., 2015). Concentrations of 50%, 40%, 30%, 20%, 10%, 5%, 2.5%, and 1% of the pig antigen were used in the test. In this test, the hyperimmune serum was dropped into the central well and the diluted pig antigens were filled into surrounding wells.
Results and Discussion
An attempt to produce a low-cost RaPS-Ab to detect pork adulteration was reported. In this experiment, the extraction of pig antigen from serum using CA was capable of producing a total soluble protein of 17.5 mg/mL from a total serum protein of 46.1 mg/mL. However, the true serum protein(s) contained remains unknown and warranted further analysis.
It is likely that the pig antigen utilized in current study mainly contained pig Albumin or Immunoglobulin. The extraction of pig antigen using CA was reported to retain immunoglobulin Gamma in the filtrate (Bokhout et al., 1986). Other studies reported that treatments of equine and Camel sera using CA resulted in two major proteins in the filtrates: Albumin and immunoglobulin (Morais and Massaldi, 2012; Shawki et al., 2017). Albumin and Immunoglobulin can be good candidates as markers to detect pork adulteration in raw or cooked meat products. The concentration of albumin in pork is ~5 mg/L (Moon et al., 2014) and it retains one-tenth of its affinity to immunoglobulin even after autoclaving (Lee et al., 2011). The resistance of Immunoglobulin under autoclaving is unknown, but in meatball samples boiled for 20 minutes, the pig immunoglobulin remained detectable using a polyclonal-antibody based lateral flow test (Kuswandi et al., 2017).
The study demonstrated that the pig antigen extracted with a simple method of caprylic acid precipitation was sufficient for producing an immunogenic pig-specific antigen. The AGID tests showed that the RaPS-Ab were detected on day 56 after initial immunization (Figure 1A). The specificity test showed that the antibodies reacted specifically with pork juice amongst five different meat species used in the test (Figure 1B). The AGID test further showed that the antibodies were capable of detecting pig antigen in as low as 1% of its initial concentration (Figure 1C). Another simple method to purify IgG for antigen preparation to produce anti IgG antibodies, consisted of two steps, included precipitation with saturated ammonium sulphate and an overnight dyalisis (Majidi et al., 2007; Sadeghi et al., 2018). Compared to this, the CA precipitation provides a simpler procedure in term of time consumed and resources allocated.
The final bleeding of the experimental animals yielded ~24 mL serum from the individual rabbit weighed two kilograms. The use of rabbits as the biological machine to produce the anti-pig antibodies may be sufficient to facilitate a large survey of 1,000 individual meat samples in a population when the AGID is to be used. For the production of a higher titer of antibodies in a rabbit, a longer time period of animal keeping or better use of Freud’s complete adjuvant might be needed (Bollen et al., 1996). In addition, the production of anti-pig antibodies in larger animal species using pig antigen extraction described in this study, as an alternative to producing lower cost and more efficient anti-pig antibodies, is open for investigations.
The affinity of the pig antigen in meat products to its specific antibodies however, can be influenced by various seasoning substances (Moon et al., 2014). On the other side, the cross-reaction of the anti-pig antibodies to different types of marinations remains unknown and further study is needed to answer this question. Further study is also advisable to develop a rapid lateral flow test described earlier (Kuswandi et al., 2017) using antibodies developed by the current method.
In conclusions, this study provides a proof of concept that pig antigen extracted from pig serum by the simple step of impurities precipitation using CA is capable of inducing pig species-specific antibodies in rabbits and the antibodies have the potential as a sensitive tool to detect low level of pork adulteration or contamination in various raw or cooked meat products.
Acknowledgements
This study is funded by The Institute of Research and Community Services (LPPM) Universitas Brawijaya, through contract number 436.37/UN10.C10/PN/2020.
Novelty Statement
To the best of our knowledge, this is the first report of the use of simple Caprilic acid extract of the pig serum as a polyvalent antigen for the production of specific antibodies to detect pig species in a meat product.
Author’s Contribution
WN conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, supervision, validation, visualization, writing-original draft, writing review and editing. K, SM, FAR and FOP data curation, investigation, methodology, project administration, resources, software, visualization, writing-original draft. MAL data curation, formal analysis, funding acquisition, methodology, resources, supervision, writing original draft, writing review and editing. SA conceptualization, formal analysis, methodology, resources, supervision, visualization, writing original draft, writing review and editing.
Conflict of interest
The authors have declared no conflict of interest.
References