Advances in Animal and Veterinary Sciences
Research Article
Role of Bovine Amniotic Membrane in Repair of Abdominal Muscle Defects in Dogs
Rania Mourad, Abdelbasit M. Abdelaal, Shimaa A. Ezzeldein, Walid Refaai*
Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Sharkia Province, Egypt.
Abstract | Repair of abdominal muscle defects using polypropylene mesh (PP) in dogs was first described in 1958. Later, other types of meshes were developed, and new techniques were used to overcome the serious complications caused by PP. The current study aimed to experimentally evaluate the use of PP together with bovine amniotic membrane (BAM) or omentalization as an anti-adhesive barrier during surgical correction of created full thickness ventral abdominal muscle defects in dogs. Twenty-four healthy dogs were divided equally into three groups. A circular full-thickness ventral abdominal wall defect about 5 cm in diameter was created in the muscles and peritoneum. In group A, PP mesh alone was used for hernioplasty, whereas in group (B) PP was combined with BAM and in group (C) dogs were treated with PP and omentalization. All groups were grossly checked for adhesions at 14 and 28 days post-surgery. Data were statisatically analyzed by SPSS. Adhesions and inflammation were significantly lower in the BAM group when compared to the PP group. Histopathologically, strong significant positive correlations were found between the degree of adhesion and inflammation scores. The use of BAM with PP was better for the repair of herniation in dogs than the use of either PP mesh alone or PP with omentalization as it was characterized by minimal adhesions and less inflammation.
Keywords | Adhesion, Bovine amniotic membrane, Hernia, Omentum, Polypropylene mesh
Received | August 25, 2020; Accepted | November 27, 2020; Published | January 01, 2021
*Correspondence | Walid Refaai, Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Sharkia Province, Egypt; Email: Walidrefaai@gmail.com
Citation | Mourad R, Abdelaal AM, Ezzeldein SA, Refaai W (2021). Role of bovine amniotic membrane in repair of abdominal muscle defects in dogs. Adv. Anim. Vet. Sci. 9(2): 182-188.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.182.188
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mourad et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Abdominal wall hernia repair using polypropylene mesh (PP) in dogs was first described by Usher and Wallace (1958). Since then, hernioplasty was popularized, other types of meshes were developed, and new techniques were used to overcome the serious complications caused by PP such as recurrence, bowel adherence and obstruction, fistula formation, wound infection, and seroma/hematoma (Lasonoff and Sauter, 2004).
Adhesions are defined as fibrous structures in the abdominal cavity that arise at injured peritoneal surfaces as a consequence of disturbed tissue repair after peritoneal trauma. To prevent adhesion formation, during hernioplasty, a mechanical barrier is used to cover the mesh over the hernia. This is combined with systemic injections of anti-inflammatory drugs, antihistamines, and fibrinolytic agents such as citrate or heparin. Ideally, the barrier lasts for at least seven days until the completion of the healing process and is then absorbed by the body (Farquhar et al., 2000).
Amniotic membrane (AM) has many attractive properties to be used as a barrier for hernia repair. It acts as a new healthy substrate that facilitates migration of epithelial cells, reinforces adhesion of basal epithelial cells, promotes epithelial differentiation, and prevents epithelial apoptosis. Additionally, it produces various growth factors that can stimulate epithelialization and reduce scar formation. The AM also inhibits protease activity, in addition to anti-inflammatory, anti-angiogenic and anti-fibrotic effects (Baradaran-Rafii et al., 2007).
Omentalization is used to seal the defect, reduce the risk of visceral adhesions, and bring blood supply and healing cells to hernia site (Engelsman et al., 2007). Omentum also has the ability to adhere to intra-abdominal foreign bodies and will surround and occlude them within seven days in dogs (Shimotsuma et al., 1994).
Omentum can be used for reconstructive purposes due to its angiogenic activity in adjacent structures. It produces omental microvascular endothelial cells which express the angiogenic peptide “basic fibroblast growth factor” (Bikfalvi et al., 1990). The process of neovascularization allows the omentum to provide vascular support to adjacent tissues such as the gut and to promote functioning and healing in ischemic or inflamed tissues (Williams et al.,1996).
Previous studies emphasized the use of human AM in preventing adhesions during repair of abdominal wall defect and in bladder reconstruction in rats (Barski et al., 2017; Liu et al., 2020). Moreover, AM graft was successfully used in the treatment of Complex vesico-vaginal fistula through an abdominal approach without either adverse events or rejection (Price and Price, 2016). In dogs, AM was used for the treatment of Complicated Corneal Ulcers and in repair of gastric mucosal defects with high satisfactory cosmetic and visual outcomes (Farghali et al., 2017; Costa et al., 2019).
As far as we knew, there is no available literature about the use of BAM in repair of abdominal muscle defects in dogs. So, the aim of the current study was to evaluate the use of PP together with BAM or omentaliztion as an anti-adhesive barrier during surgical correction of ventral abdominal wall defect in dogs.
MATERIALS AND METHODS
Study design
This work was performed at Department of Surgery, Anesthesiology, and Radiology after obtaining permission from the committee for animal welfare and care at the Faculty of Veterinary Medicine, Zagazig University, Egypt.
Twenty-four healthy male mongrel dogs (average age: 1.5–2 years and average weight: 15–20 kg) were included in this study. Dogs were given anthelmintics (Revolution®, Zoetis Inc. USA) two weeks before surgery.
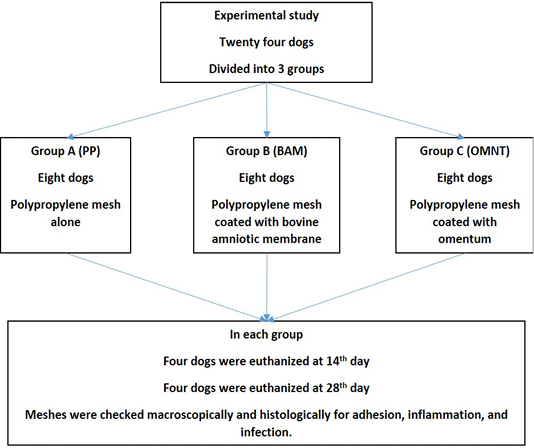
Dogs were divided into three groups, eight dogs for each. In group A (PP Group): PP alone was used, whereas in group B (BAM Group): PP was combined with BAM and in group C (OMNT Group) dogs were treated with PP and omentalization. Within each group, four dogs were euthanized 14 days post-surgery and the other four at 28th day post-surgery (Figure 1).
Animals were checked for adhesions, inflammation, and wound infections through laparoscopy and/or by direct examination after euthanasia at either 14 or 28 days post-surgery. Concerning laparoscopy, only one port was performed in ventral abdominal wall for insertion of the scope for evaluation of the intra-abdominal condition. The site of port placement was about 5 cm cranial to the umbilicus to visualize the defect site (Fiobianco et al., 2012). Tissue specimens were collected and preserved in 10% formalin and stained by HandE for histological examination (Suvarna et al., 2013).
Collection and preparation of bovine amniotic membrane
BAM was obtained from the placentae of Friesian cows within 2 h after parturition. These placenta were washed three times with normal saline 0.9% followed by povidone iodine solution 0.1%, and then with gentamycin solution 0.2% and stored in 98% glycerin at room temperature for an average of 66 days before use (Plummer, 2009). A sample of the membrane was mounted in 10% formalin and submitted for histopathological examination to check its viability, while a second sample was submitted for bacterial culture to confirm asepsis.
Surgical techniques
Dogs were injected with Cephalosporin (20 mg/kg bwt, Cefaxone-pharco Co., Cairo, Egypt) 24 h before surgery, and food and water were withheld for 12 h and 4 h, respectively before surgery. Thirty minutes prior to surgery, animals were premedicated with Atropine sulfate (0.04 mg/kg bwt, IM, Misr Co, Cairo, Egypt) followed by Xylazine HCl (1 mg/kg bwt, IM, Xylaject 2% -ADWIA, Egypt). General anesthesia was induced and maintained using Thiopental sodium 2.5% (20 mg/kg bwt, IV, Anapental Sod., Segmatec, Egypt). Animals were placed in dorsal recumbency, and the skin of the ventral abdominal region and both flank regions was prepared aseptically from xiphoid to the pubic symphysis.
A longitudinal midline incision (10 cm in length) was made in the skin at the ventral midline of the abdomen starting anterior to the umbilicus by 3–4 cm and extended caudally just cranial to preputial orifice with blunt dissection of the subcutaneous tissue. A circular full-thickness ventral abdominal wall defect including muscles and peritoneum with a diameter of 5 cm at the mesogastric region was created, starting cranially above the umbilicus and extending caudally. The circular defect transformed directly into an elongated oval shape due to tension forces exerted by the surrounding abdominal muscles.
In group A, PP mesh (15 x 15 cm, Ethicon, Cincinnati, Ohio State, USA) was trimmed to the shape of the defect, placed in direct contact with the viscera and sutured with interrupted mattress sutures using 2/0 polypropylene suture material applying the underlay technique (Schumpelick et al., 2006). In group B, BAM was tailored to fit the defect, placed in direct contact with the viscera and sutured together with the mesh using the same technique as in group A. In group C, omentalization was done by fixing a patch of omentum from the same animal within the margin of the surgical defect using the interrupted mattress technique such that the omentum was in direct contact with visceral organs and separating them from the PP. The procedure was completed as in group A. Finally, subcutaneous tissue and skin were closed over the implants in all animals.
Animals were injected with Ketoprofen (Amriya Co., Alexandria, Egypt) at a dose of 1 mg/kg bwt directly after surgery and once daily for three successive days. Cephalosporin (20 mg/kg bwt, Cefaxone-pharco Co., Cairo, Egypt) was administered for five successive days at 12 hour intervals. Povidone iodine was applied to the wound twice daily. Dogs were fed on reduced amounts (one third of the regular diet) of soft food for the first week postoperatively and gradually returned to normal amounts within two weeks. The skin stitches were removed after two weeks.
Animals were observed daily for presence or absence of postoperative complications such as seroma, hematoma, stitch abscesses, or wound dehiscence.
Dogs were euthanized at 14 and 28 days post-surgery by means of large doses of Thiopental sodium. Operation sites were checked for adhesions and inflammation, and results were recorded according to an adhesion scoring system (Deeken and Mathews, 2012) by which adhesion was classified qualitatively into four grades (Grade 0: Absence of adhesion between mesh and viscera, Grade 1: Minimal adhesion that could be freed by gentle hand dissection, Grade 2: Moderate adhesion that could be freed by aggressive hand dissection, and Grade 3: Dense adhesion that required sharp dissection).
Tissue specimens were collected from the operation site, preserved in 10% formalin, stained with H and E and checked for inflammation, neovascularization, and fibrous tissue development, which was classified according to a semi-quantitative grading scale (Grade 0: no response, Grade 1: minimal/barely detectable, Grade 2: mild/slightly detectable, Grade 3: moderate/easily detectable, Grade 4: marked/very evident) (Deeken and Mathews, 2012).
Statistical analysis
Data were statistically analyzed using SPSS software. Analysis was performed by the Kruskal-Wallis rank sum test. Multiple comparisons testing was performed using Dunn´s test with Bonferroni correction. Results were represented as median (IQR). Spearman’s rank correlation was used to investigate the relationships between experimental groups. In all cases, a P-value < 0.05 was considered significant.
RESULTS AND DISCUSSION
Large abdominal wall defects have always been a problem to deal with both in veterinary and human medicine due to the many associated complications including seroma, hematoma, recurrence, adhesion, and even death (Melman et al., 2010). Moreover, insufficient autogenous tissue for adequate abdominal wall closure remains a significant problem for surgical repair of large abdominal wall defects making the use of biomaterials unavoidable (Lai et al., 2003).
Biomaterials used for abdominal repair achieve tension-free repairs and result in significant reductions in postoperative pain, shorter recovery periods, and lower incidence of recurrence (Amid, 1997). Kalaba et al. (2016) came to the conclusion that although there is huge variety of mesh products available, they all have limitations, and no ideal mesh, which will prevent adverse effects, currently exists.
PP is the most common mesh type used during herniorrhaphy (Cevasco and Itani, 2012). However, the use of PP alone has been shown to result in a greater adhesions compared to the use of composites made from polypropylene-based meshes paired with other protective barriers (Novitsky et al., 2007).
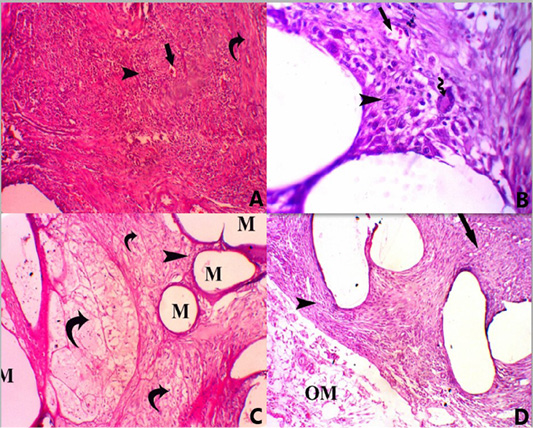
Figure 2: A photomicrograph of all groups at 14 days post-surgery. (A) Group A, showing PP mesh surrounded by marked infiltration of mononuclear inflammatory cells (score 4) (head arrow) with mild neovascularization (score 2) (straight arrow) and mild fibrosis (score 2) (Curved arrow), H and E, stain. X 100. (B) Foreign body giant multinucleated cell (score 1) (curved arrow), H and E, stain. X 400. (C) Group B, showing mesh (M) surrounded by minimal infiltration of mononuclear inflammatory cells (score 1) (head arrow) with persistence of vital AM (curved arrow) H and E, stain. X 100. (D) Group C, showing mesh surrounded by moderate infiltration of mononuclear inflammatory cells (score 3) (head arrow) with mild fibrosis (score 2) (straight arrow) and persistence of omentum (OM) next to the mesh (score 2), H and E, stain. X 100.
A previous study indicated that an ideal, adhesion-free peritoneal interface could be achieved using a composite composed of a polypropylene mesh with a laminar surface positioned in contact with the visceral peritoneum (Amid, 1997). Composite barrier meshes consist of two separate layers, a main structural mesh and an anti-adhesive layer. These two layers may be stitched or vacuum-pressed together (Deeken and Lake, 2017).
In this study, the mesh was secured using the underlay technique, which had the advantage of providing excellent incorporation into the abdominal wall with sufficient protection of the viscera and greater security at the mesh fascia interface. Additionally, no herniation was observed in any of the animals in our study, which was one of the preferred outcomes of this technique. These findings were supported by previous studies (Berhanu and Talbot, 2015; Karrouf et al., 2016).
Higher degrees of adhesion formation were encountered in animals where defects were repaired with PP alone (PP Group, Table 1, Figure 2). This could be attributed to the direct contact between the PP and the abdominal viscera leading to an inflammatory response to the prosthesis and giving rise to intra-abdominal adhesion (Silva et al., 2010).
Table 1: Score of inflammation, neovascularization, and fibrosis and degree of adhesion in all experimental groups at 14th and 28th days post-surgery according to a semi-quantative grading scale described by Deeken and Mathews (2012).
| Groups | Group A (PP only) | Group B (PP+BAM) | Group C (PP+omentum) | |
| Inflammation score |
14th day |
3.5 (1.00)a |
1.00 (0.25)b |
2.50 (1.25)ab |
|
28th day |
3.00 (0.25)a |
1.5 (1.00)b |
2.0 (0.00)ab |
|
| Neovascularization score |
14th day |
2.00 (0.25)a |
3.00 (0.25)a |
2.5 (1.00)a |
|
28th day |
1.50 (1.00)a |
2.00 (0.25)a |
1.0 (0.00)a |
|
| Fibrosis score |
14th day |
1.5 (1.00)a |
2.0 (0.00)a |
2.0 (0.50)a |
|
28th day |
3.00 (0.25)a |
2.00 (0.25)a |
3.00 (0.50)a |
|
| Degree of adhesion |
14th day |
3.00 (0.25)a |
0.00 (0.25)b |
1.50 (1.00)ab |
|
28th day |
3.00 (0.00)a |
0.00 (0.25)b |
1.00 (0.50)ab |
|
Data were presented as median (IQR); Differences between groups with different letters are significant (P value < 0.05).
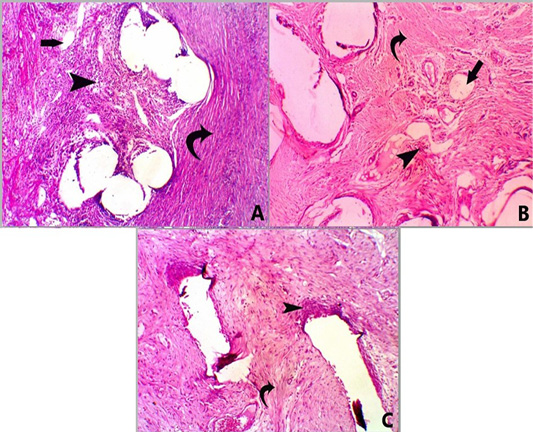
Figure 3: A photomicrograph of H and E stained sections for all groups at 28 days post-surgery. Group A, showing mesh surrounded by moderate infiltration of mononuclear inflammatory cells (score 3) (head arrow) with moderate fibrosis (score 3) (curved arrow) and minimal neovascularization (score 1) (straight arrow) X 100. Group B, showing mesh surrounded by mild infiltration of mononuclear inflammatory cells (score 2) (head arrow) with moderate fibrosis (score 3) (curved arrow) and minimal neovascularization (score 1) (straight arrow), X 100. Group C, showed mesh surrounded by mild infiltration of mononuclear inflammatory cells (score 2) (head arrow) with marked fibrosis (score 4) (curved arrow) X 100.
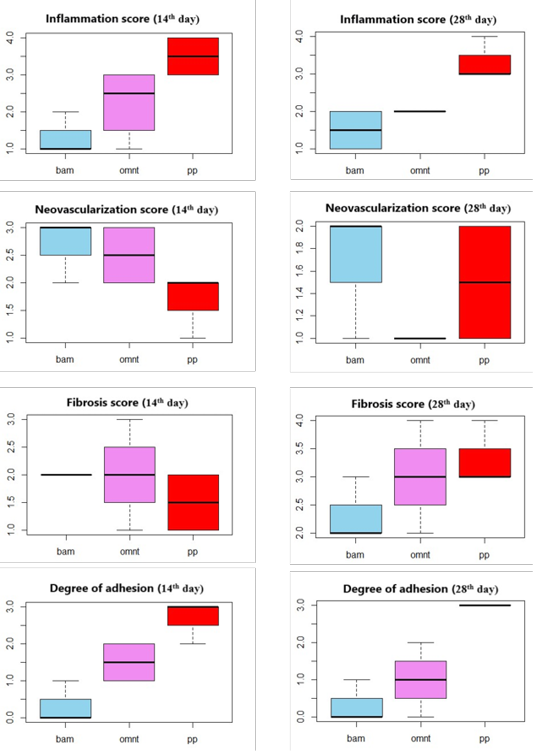
Figure 4: Score of inflammation, neovascularization, and fibrosis and degree of adhesion in all experimental groups at 14th and 28th days post-surgery according to a semi-quantative grading scale described by Deeken and Mathews (2012).
Clear abdominal viscera and lesser or no adhesions between mesh sites and visceral organs were observed in BAM Group (Table 1). Amniotic Membrane has been shown to have anti-inflammatory and antifibrotic action through suppression of pro-inflammatory cytokines. It also has antibacterial and antiviral effects due to the presence of various compounds that promote anti-microbial immunity. It acts as a new healthy substrate that facilitates migration of epithelial cells, reinforces adhesion of basal epithelial cells, promotes epithelial differentiation, and prevents epithelial apoptosis. It also produces various growth factors that can stimulate epithelialization and reduce scar formation (Plummer, 2009). Vital human AM has been shown experimentally to provide excellent anti-adhesion effects. In addition to its superior handling characteristics, it also showed good biocompatibility and rapid integration (Petter-Puchner et al., 2011).
In our study, minimal degrees or no adhesion were recorded in the BAM group proving that the BAM is suitable as an anti-adhesive material used in combination with prosthetic mesh for hernia repair (Figure 3). These results were in agreement with previous work (Petter-Puchner et al., 2011). The use of processed BAM as a xenograft without rejection or any side effects of immunogenicity has previously been demonstrated in dogs (Ramuta and Kreft, 2018).
The OMNT Group had non-significant lower adhesion scores than PP Group, but higher scores than BAM Group (Table 1, Figure 4). However, it is possible that normal attachment of the omentum to the viscera might be misdiagnosed as adhesion. Furthermore, mesothelial cells within the omentum secrete fibrinolytic factors which have antifibrotic effects (Giusto et al., 2018). Hence, the lower adhesion observed in group C can be attributed to the nature and action of the omentum. Thus, our results indicate that the use of omentum as a mechanical barrier between the injured abdominal wall and the viscera appears to be preferable to the use of PP alone.
The highest degrees of inflammation and adhesions were recorded in PP Group, followed by OMNT Group as reported by Hu et al. (2020). However, the BAM group had the lowest inflammation with the absence giant cells accompanied by good neovascularization and collagen deposition indicating excellent implant integration (Rashid et al., 2018; Soylu et al., 2018) (Table 1, Figures 2-4).
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, large abdominal wall defects have always been a problem to deal with both in veterinary and human medicine due to insufficient autogenous tissue for adequate abdominal wall closure. Biomaterials used for abdominal repair achieve tension-free repairs and result in significant reductions in postoperative pain, shorter recovery periods, and lower incidence of recurrence. Thus, the use of PP alone resulted in a lot of adhesions and more inflammation. While, the use of antiadhesive layer, BAM and Omentum respectively, with PP mesh yielded better results as it was characterized by minimal adhesions and less inflammation.
ACKNOWLEDGMENTS
The authors are grateful to all staff members at the Department of Surgery, Anesthesiology, and Radiology, Faculty of Veterinary Medicine, Zagazig University for their support. Many thanks for Dr. Mohammed Al Abyad (Lecturer of Pathology, Faculty of Medicine, Zagazig University) for the histopathological work. Special thanks for Dr. Shebl Salem (Lecturer of Surgery, Faculty of Veterinary Medicine, Zagazig University) for statistical analysis.
Author’s Contribution
Mourad, R. participated in study design, performed experimental part, interpreted the results and drafted the manuscript. Abdelaal, A. M. Participated in the study design, supervised experimental work, and revised the manuscript.Ezzeldein, S. A. participated in study design and interpretation of the results. Refaai, W. participated in the study design, supervised experimental work, analyzed the data, and revised the manuscript. All authors approved the final manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES