Advances in Animal and Veterinary Sciences
Case Report
Bone Marrow Metastasis in Inflammatory Mammary Carcinoma: A Case Report in Dog
Miriã R. de Oliveira1, Tatiany L. Silveira1, Rubens A. Carneiro2, Gleidice E. Lavalle2, Michele A. Rodrigues1, Geovanni D. Cassali1*
1Laboratory of Comparative Pathology, Department of General Pathology, Biological Sciences Institute, Federal University of Minas Gerais; 2Veterinary Hospital, Veterinary School, Federal University of Minas Gerais.
Abstract | We report four cases of disseminated tumor cells (DTCs) present in the bone marrow (BM) of canines with inflammatory mammary carcinoma (IMC). The diagnosis was made by cytological and histopathological analysis of BM biopsy and was confirmed by immunohistochemistry and immunofluorescence methods using an anti-cytokeratin AE1/AE3 antibody. The cytologic evaluation followed by BM immunocytochemistry is a widely used method for the investigation of DTCs in women with breast neoplasms. Few studies have reported the presence of these cells in dogs, including IMC. The presence of these cells in the BM of women with breast tumors is related to tumor recurrence. This study demonstrates the presence of DTCs in the BM of dogs with IMC. Furthermore, the observation of the presence of micrometastasis at the time of diagnosis combined with cytokeratin immunocytochemical analysis could be a valuable prognostic tool in veterinary patients.
Keywords | Bone marrow, Disseminated tumor cells, Metastasis, Inflammatory carcinoma, Dog
Received | May 22, 2020; Accepted | July 15, 2020; Published | August 10, 2020
*Correspondence | Geovanni Dantas Cassali, Laboratório de Patologia Comparada, Departamento de Patologia Geral, Instituto de Ciências Biológicas (ICB), Universidade Federal de Minas Gerais. Avenida Presidente Antônio Carlos, 6627. ZIP Code: 31270-901. Belo Horizonte, Minas Gerais, Brazil; Email: cassalig@icb.ufmg.br
Citation | Oliveira MR, Silveira TL, Carneiro RA, Lavalle GE, Rodrigues MA, Cassali GD (2020). Bone marrow metastasis in inflammatory mammary carcinoma: A case report in dog. Adv. Anim. Vet. Sci. 8(10): 1087-1090.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.10.1087.1090
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Oliveira et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The inflammatory mammary carcinoma (IMC) is the clinical-pathological presentation of a local invasion and extremely aggressive mammary neoplasia (Peña et al., 2003). Currently represents one of the biggest challenges in veterinary oncology, considering its high metastatic potential, hyperacute course, and poor prognosis (Janni et al., 2011).
In veterinary and human oncology, metastatic disease is one of the leading causes of death in patients, especially in those with solid carcinomas (Argyle, 2003). In 2002, a new model of metastasis was proposed, in which the cancer cells acquired metastatic capacity during the initial stages of the primary tumor (Rene and Weinberg, 2002). When these cells are found in bone marrow (BM), they are called disseminated tumor cells (DTCs) (Pantel et al., 1993). Previous studies demonstrated that persistent DTCs during follow-up in women with a primary diagnosis of early breast cancer predicted relapse and death significantly, and these observations provide strong evidence for its value as a monitoring tool (Janni et al., 2011; Taylor et al., 2013). Finally, a meta-analysis evaluating patients with primary breast cancer demonstrated that the presence of circulating tumor cells could be associated with poor overall survival and biologically more aggressive phenotypes (Zhao et al., 2011). Cytologic examination of BM is not a routine part of staging in veterinary patients with epithelial tumors. The micrometastasis presence at the time of diagnosis has not been reported as a prognostic tool in veterinary patients. This study aimed to evaluate the presence of DTCs in BM of dogs with IMC.
For immunohistochemistry, the gelatinized slides containing the 4-μm histological sections were deparaffinized in xylol and rehydrated in a progressively diluted alcohol series. Antigen retrieval reactions were performed under pressurized moist heat (Pascal®) with citrate buffer pH 6.0 (Dako Cytomation Target Retrieval Solution) at 125ºC for 2 minutes. After antigen retrieval, the slides were cooled for 20 minutes, followed by three 5-minute washes in PBS. Peroxidase and endogenous protein blocking were performed with three PBS washes between each blocking step. Subsequently, all histological sections were coated with the primary mouse monoclonal antibody against Pan-Cytokeratin (1:500, clones AE1/AE3, Dako North America, Carpinteria, California, USA) and incubated in a humidified chamber for 16 hours. After the incubation period, three PBS washes were performed, and the secondary antibody was applied to the histological sections, which was followed by washing in PBS, and then the addition of the polymer. Colorimetric development was performed using diaminobenzidine (DAB) for 3 minutes. Sections were counterstained in Harri’s hematoxylin (H-E). All reagents from Novo Link Kit (Leica®) were used according to the manufacturer’s instructions. For controls were used mammary carcinoma samples, and the negative control was omitting the primary antibody.
Immunofluorescence staining was performed as previously described by Rodrigues et al. (2016). Cells were labeled with a mouse monoclonal antibody against Pan-Cytokeratin conjugated to Alexa Fluor 488 (1:250, clones AE1/AE3, Sigma-Aldrich, Carlsbad, California, USA) overnight at 4ºC and Hoechst 33258 (1 mg/mL, Life Technologies) for 1 hour at room temperature. Next, samples were washed three times in PBS for 10 min and then mounted with Hydromount (Electron Microscopy Sciences). The primary antibody was omitted in all negative controls. Images were collected using a Zeiss LSM 880 confocal microscope (Carl Zeiss, Jena, Germany) using a 40× 1.3 NA oil objective lens and a GaAsP detector. Samples were excited at 405 nm and observed at 415-480 nm to detect Hoechst and at 510-540 nm to detect Alexa 488. The software Zeiss Efficient Navigation (ZEN) was used for fluorescence analyses and image adjustments according to the negative control (Rodrigues et al., 2016). For all negative controls were used BM smears from the same dogs (Figure 2).
Four female dogs were clinically diagnosed with inflammatory mammary carcinoma at “Serviço de Oncologia do Hospital Veterinário da Universidade Federal de Minas Gerais (SO-UFMG)” and submitted to fine-needle aspiration (FNA) at the moment of the clinical diagnostic. All dogs presented the secondary form of the disease; they developed the IMC during the recurrence of the mammary tumor and also presented metastasis in lymph nodes and distant organs. When the dogs presented primary mammary neoplasia, they were forwarded to surgery. The tumor was removed and sent to “Laboratório de Patologia Comparada do Instituto de Ciências Biológicas (LPC-ICB) da UFMG” for the diagnostic (Elston, 1991; Misdorp et al., 1999; Goldschmidt et al., 2011; Cassali et al., 2014).
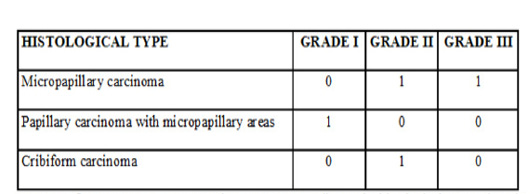
The clinicopathological findings are represented in Table 1.
The local for the FNA was determined according to the size of the patient. In medium and large dogs, the biopsy was performed in the sternum manubrium; and in small dogs, it was made on the iliac crest. The asepsis and anaesthesia were made in the local of the biopsy. It was inserted an 18G needle with a 10 mL syringe into the bone by pressure and using clockwise and counter clockwise movements until it was firmly fixed. The BM aspiration was performed, and the material was deposited in slides for the squash and Giemsa staining (Taylor et al., 2013). The BM microscopic screening method of all samples was done, and it was analyzed the presence of epithelial-like cells, that is suggestive of DTCs. The presence of these cells was observed in four samples of BM (Figure 1A).
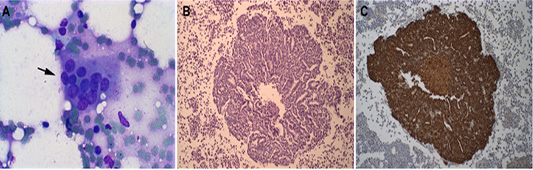
Figure 1: Representative Bone marrow photomicrographs: (A) Epithelial cell clusters in the smear are compatible with DTCs from inflammatory mammary carcinoma (see black arrow). Giemsa staining, obj. 10×. (B) Histopathology of canine bone marrow with the presence of epithelial cells in a cribriform tumor (H-E), obj. 20×. (C) Cytokeratin positive staining in epithelial cells present in canine bone marrow, counterstained in Harri’s hematoxylin, obj. 20×.
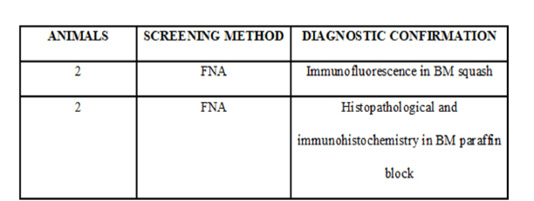
All four dogs in this study died due to the evolution of the disease. Concerning overall survival time ranged from 3 to 20 days (median 16 days). The necropsy was performed in two animals, and BM samples were collected to histopathological and immunohistochemistry analyses for DTCs (Figure 1B and 1C). The BM samples were fixed in 10% neutral-buffered formalin for 48 h, processed by the routine paraffin method, and stained with hematoxylin-eosin. The owners of the two other animals did not allow the necropsy. The BM squash previously performed was used for immunofluorescence (Figure 2). Immunofluorescence in bone marrow smear and paraffin-embedded tissue confirmed the epithelial cells subcellular localization with the cytokeratin staining. The number of animals, screening methods and the technique used for the diagnostic confirmation are presented in Table 2.
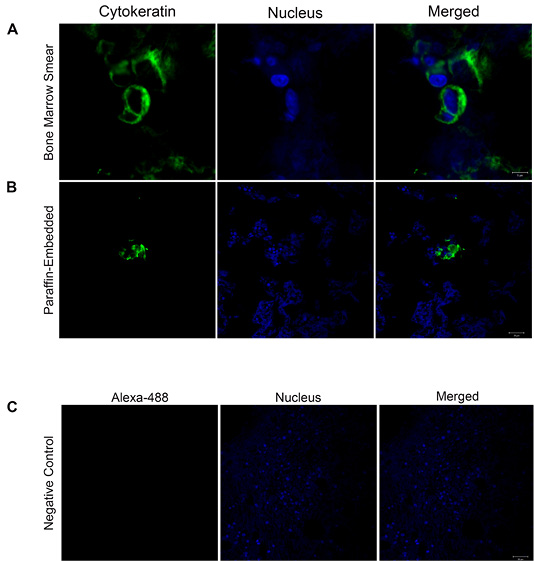
Figure 2: Cytokeratin localization confirmed by confocal microscopy: (A-B) Immunofluorescence images show in green cytokeratin localization in bone marrow smear and paraffin-embedded tissue (Scale Bars= 5 and 20 µm). The nuclei were stained with Hoechst and are shown in blue. The merged images confirm the subcellular localization of cytokeratin. (C) Representative image of the negative control. Images are representative of what was observed in two independent experiments. Scale Bar: 20 µm.
Our study describes the presence of DTCs coming from IMC in dogs. Previous studies showed the presence of micrometastases in BM in animals with apocrine adenocarcinoma of anal sac gland, apocrine carcinoma of the skin, and presence of DTC’s in no inflammatory mammary neoplasia (Jaillardon et al., 2012; Taylor et al., 2013). Some authors suggest that neoplastic cells affinity to BM is due to the hematopoietically substances active, which make the microenvironment favorable to its survival and latency. The presence of these cells in BM in women with breast cancer could explain the disease recurrence after a long period since standard cytotoxic agents are not active in resting cells. After the end of conventional chemotherapy treatment, the cells return to activate and migrate to new metastatic foci (Hu et al., 2017).
The presence of DTCs in BM is well established as a poor prognostic indicator to free-survival disease and overall survival in women’s mammary cancer (Braun et al., 2005). We believe that the presence of DTCs in canine IMC does not change the prognosis because it is an aggressive and hyperacute course disease. Currently, studies are focused on the analysis of the DTCs as a clinical monitoring tool because of the increased risk of recurrence in patients with DTCs in BM. These patients could be benefited from additional treatment with adjuvant chemotherapy since conventional therapy does not work in these cells (Braun et al., 2005; Janni et al., 2011). Therefore, the identification of DTCs can lead to new therapeutic approaches to better control the disease and overall survival (Ito et al., 2012). In veterinary medicine, research related to DTC still scarce and insufficient to infer the influence on the prognosis in mammary carcinoma in dogs.
In this study, all animals presented advanced clinical staging. Previous work reported the presence of DTCs in patients with initial clinical-stage, so further studies are needed to establish the importance of DTCs in tumor progression. In veterinary medicine, the role of DTCs should be investigated in canine mammary neoplasms, since they may be an auxiliary, low invasive, and low-cost tool for understanding the progression of the disease.
Acknowledgments
This work was supported by grants from CNPq, FAPEMIG, and CAPES. The microscopic data shown in this work were obtained using the microscopes of “Centro de Aquisição e Processamento de Imagens” (CAPI -ICB/UFMG).
Authors Contributions
All authors contributed to the study conception and design. Geovanni D. Cassali, Miriã R. de Oliveira, Tatiany L. Silveira, and Michele R. Rodrigues designed the experiments and wrote the paper. Miriã R. de Oliveira, Tatiany L. Silveira, and Michele R. Rodrigues performed the experiments. Geovanni D. Cassali, Rubens A. Carneiro, and Gleidice E. Lavalle performed the cytopathological data analysis. All authors read and approved the final manuscript.
Ethics approval
All procedures performed in studies involving animals were following the ethical standards and were approved by the Animal Research Ethics Committee of Universidade Federal de Minas Gerais, Brazil (n° CEUA 200/2016).
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES