Advances in Animal and Veterinary Sciences
Research Article
The Placenta Anatomy of Sunda Porcupine (Hystrix javanica)
Teguh Budipitojo*, Siti Shofiyah, Dian Bekti Hadi Masithoh, Linda Miftakhul Khasanah, Irma Padeta
Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia.
Abstract | Sunda porcupines (Hystrix javanica) belongs to the Order of Rodentia is an endemic animal of Indonesia. The aims of this study are to determine the types and histological structure of Sunda porcupine placenta. Data regarding the type and histological structure of Sunda porcupines’ placental organs can be used to support the conservation of Sunda porcupines. This research used two samples of placenta Sunda Porcupine from Ngawi, East Java. Placenta were fixed in Bouin’s solution for 24 hours. Tissues were processed by using paraffin method and cut in 5 µm thickness. Tissues slide were stained with Hematoxylin Eosin (HE) to identified histological structure of the Sunda porcupine placenta. Photomicrographs were using Optilab Image Viewer. The histological structure of the Sunda porcupine’s placenta were analyzed and reported descriptively. Macroscopically, the shape of Sunda Porcupine placenta is flat like a disc. Histologically, the thickest parts of Sunda porcupines placenta consist of chorioallantoic plate, labyrinth zone, trophospongium, decidua, metrial glands, myometrium zone. In conclussion, the placenta of sunda porcupine (Hystrix javanica) has been identified which has a discoid shape and is classified to the hemochorial type.
Keywords | Sunda porcupines, Placenta, Type, Histological structure
Received | November 21, 2019; Accepted | February 17, 2020; Published | March 03, 2020
*Correspondence | Teguh Budipitojo, Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia; Email: budipitojo@ugm.ac.id
Citation | Budipitojo T, Shofiyah S, Masithoh DBH, Khasanah LM, Padeta I (2020). The placenta anatomy of sunda porcupine (Hystrix javanica). Adv. Anim. Vet. Sci. 8(3): 223-228.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.223.228
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Budipitojo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Porcupines are unique mammals belonging to the Order of Rodentia, the suborder of Hystricomorpha, and family of Hystricidae. Porcupines have small head size, small ears, short tail, and short legs that are strong for foraging by digging (Michael et al., 2004). The upper part of the porcupine’s body is covered with stiff cylindrical spines, black-ringed or dark brown and white, while the lower part of the body is overgrown with slightly coarse black hair. Thorns in a porcupine’s body are a modification of his hair. This animal is active at night, while throughout the day it stays in its nest inside the ground. In its natural habitat, porcupines like tubers, rhizomes, forest fruits, leaves, young stems, roots, buds, internal stems, roots, bark, and bamboo shoots (Farida and Ridwan, 2011; Khan et al., 2019). Sunda porcupine can be found in Indonesia around Java, Bali, Sumbawa, Flores, Lombok, Madura, Nusa Tenggara, South Sulawesi, and Southeast Sulawesi (Weers, 1979).
Porcupine is one of the protected animals in Indonesia. According to the Regulation of the Minister of Environment and Forestry of the Republic of Indonesia Number P.92/Menlhk/Setjen/Kum.1/8/2018 concerning Types of Protected Plants and Animals, Sunda porcupines (Hystrix javanica) are included in the list of protected animals. According to Lunde and Aplin (2008), the International Union for the Conservation of Nature (IUCN) in 2008, stated that porcupines as animals with the least concern category meant that there was still plenty of availability in nature and few threats. In reality, illegal hunting of porcupines continues to increase. People in several regions in Indonesia believe that porcupine meat and other parts of the body contain medicinal properties. Also, porcupine spines used for ornamental purposes. Thus, it can be concluded that the presence of porcupine is lessen marked by scarcity of Javanese porcupines in the wild (Farida and Ridwan, 2011).
A Placenta is an apparatus for exchanging substances between the parent and the child and vice versa. Placenta serves to connect the parent and child (Andriyani et al., 2015). The component structure of placenta rodents is divided into two, namely the fetal part and the maternal part. The fetal part itself consists of the basal zone, labyrinth zone, yolk sac while the maternal part consists of metrial glands and decidua (Furukawa et al., 2011). The placenta has several functions, namely as a developing fetal barrier to the uterine wall, mediating maternal immunity tolerance, O2/CO2 gas exchange, providing nutrition for the fetus, removing waste products during fetal development, protecting the fetus to the endometrium against xenobiotics, releasing various steroids, hormones and cytokines (Furukawa et al., 2014).
Based on its shape, Placenta can be divided into four types, namely zonary, diffuse, discoid and cotyledonary types. Classification of the placenta based on the histological structure is divided into four types, namely endotheliochorial, epitheliochorial, syndesmochorial and hemochorial. Since here is no anatomical data related placenta of Sunda porcupine, the research was aimed to identify the type and histological structure of placenta of Sunda porcupine (Furukawa et al., 2014).
MATERIALS AND METHODS
Materials
This research used two placenta of Sunda porcupine from Ngawi, East Java. The samples were processed for Hematoxylin-Eosin staining method. The sample of the placenta of Sunda porcupine was obtained from porcupine hunters in Ngawi, East Java. Under the permition of Ethichal Clearance Number 326/KEC-LPPT/IX/2015, the placenta is collected and fixed in Bouin’s solution for 24 hours. The collected placenta is observed macroscopically and documented. The fixed sample is trimmed and later on put in a tissue cassette and labeled.
The placental tissues were processed by using paraffin method which consists of dehydration stage using 70%, 80%, 90%, 95% alcohol solution, absolute alcohol I, II, and III, clearing stage using xylene I, II, and III solutions, stages of paraffin infiltration using liquid paraffin I, II, and III, embedding stage, and tissue cutting stage. The tissues slide of placental samples were stained using Hematoxylin-Eosin staining method, consist of deparaffinization stage using xylene I, II, and III solution, rehydration using absolute alcohol I, II, III, 90%, 80% and 70% alcohol, hematoxylin-eosin staining stage using Harris Hematoxylin solution and eosin solution, dehydration (coverslip) using an 70%, 80%, 90%, 95% alcohol solution, absolute alcohol I, II, and III, purification (coverslip) using xylene I, II, and III solutions, and the last step is mounting (Alturkistani et al., 2016).
RESULTS AND DISCUSSION
The macroscopic photograph of the Sunda porcupine placenta shows that the shape of Sunda porcupine placenta is flat like a disc. According to Furukawa et al. (2014), placenta Rodentia, in general, is morphologically has discoid, which flat like discs, circular in shape and classified in the hemochorial type. Macroscopically, the results of this study indicate a similarity between placenta of Sunda porcupine and placenta of Rodentia in general, namely flat placenta such as discs and circular shape. The Sunda porcupine placenta consist of thickest, thin, and thinnest disc part (Figure 1).
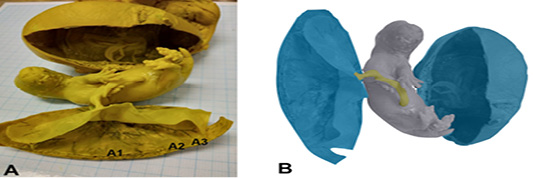
Figure 1: Photomacroscopic and schematic drawing of Sunda porcupine placenta. A is a macroscopic picture of fetal and Sunda porcupine placenta consist of thickes (A1), thin (A2), and thinest (A3) disc parts. B is a schematic drawing of A: blue is a placenta, yellow is umbilical cord, and grey is fetal.
The results of hematoxylin-eosin (HE) staining provide an overview of the histological structure of Sunda porcupine placentas in the three samples that used. Figure 2 shows an histology structure of Sunda porcupine placenta in the thickest parts (A1) according to Figure 1. The thickest part of Sunda porcupine placenta is divided into five layers to spot the distribution of villi from the maternal and fetal parts as well as other parts of the Sunda porcupine placenta. The thickest part layers of Sunda porcupine placenta consist of chorioallantoic plate, labyrinth zone, troposongoium, decidua, metrial glands, and myometrium.
The photomicroscopic of the Sunda porcupine placenta in section A1.1 or the innermost part of the Sunda porcupine placenta shows the presence of a chorioallantoic plate (Figure 3). The HE staining in Sunda porcupine placentas shows a picture of histological structure in the labyrinth region (Figure 4). The labyrinth region consists of three layers of trophoblasts, which separate the maternal blood vessels and fetal blood vessels (Furukawa et al., 2014; Bobek et al., 2018). Trophoblasts are ectodermal cells that protect the embryo and their function is in nutrition and implantation of the embryo (de Rijk et al., 2002; Furukawa et al., 2019).
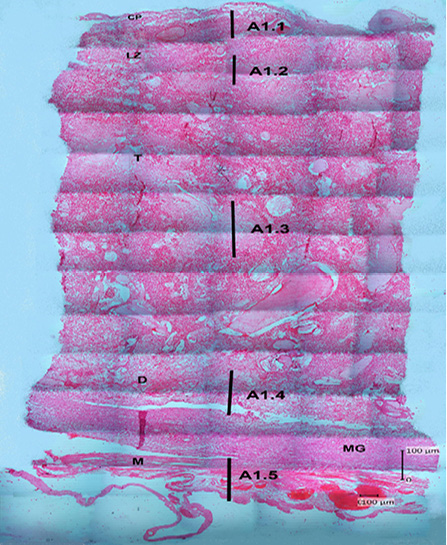
Figure 2: Photomicrograph of the thickest parts of Sunda porcupine placenta refers to Figure 1. A1 (HE). A1.1 is the deepest part, A1.2 is the second part after the innermost part, A1.3 is the middle part, A1.4 is the second outer part, A1.5 is the outermost part of the Sunda porcupine placental thickest disc. chorioallantoic plate (CP), labyrinth zone (LZ), trophospongium (T), decidua (D), metrial glands (MG), myometrium zone (M).
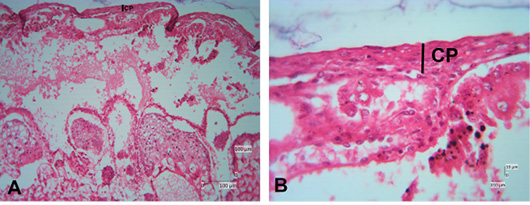
Figure 3: Photomicrograph of chorioallantoic plate area of Sunda porcupine placental disc (A1.1; HE). (A) is the low (125x), (B) are in high magnification (500 x).
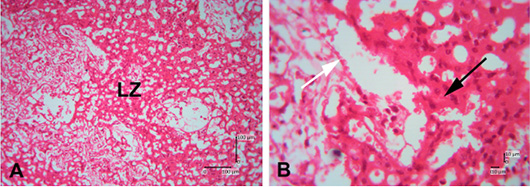
Figure 4: Photomicrograph of labyrinth zone of Sunda porcupine placental disc (A1.2; HE). (A) is in low (125 x) and (B) is in high magnification (500 x). LZ is the labyrinth zone. The white arrow shows the maternal epithelial cells that protrude to form villi. The black arrow shows trophoblast cells in the fetal part of the labyrinth zone.
The labyrinth layer is highly vascularized with fetal and maternal blood. Fetal blood vessels are limited by embryonic endothelial cells and contain immature erythrocytes (Treuting et al., 2018). Apart from being restricted to embryonic endothelial cells, it is also limited by perivascular cells, fetal mesenchymal cells, and three thin layers of trophoblast cells (cytotrophoblast and two layers of syncytiotrophoblast) (Cline et al., 2014). The maternal and fetal blood are close to each other, and most fetomaternal substance exchanges are carried out in the labyrinth zone. The labyrinth zone is a major part of the placenta development during pregnancy (Furukawa et al., 2011). The labyrinth zone is a very vascular part of the fetus. In this zone, the villi are formed from the maternal (decidual) part, which formerly was maternal endometrium tissues. The results of the analysis still cannot be distinguished between fetal capillary and maternal sinusoids. The boundaries of perivascular cells in the labyrinth zone of the Sunda porcupine are still indistinguishable.
The histological structure of Sunda porcupine placenta in section A1.2 is still part of the labyrinth zone, which looks highly vascularized with fetal and maternal blood. In this part, the villi are formed from the maternal (decidual) part and trophoblast cells (Oliveira et al., 2008; Treuting et al., 2018). In this section, there are also many vascularities between the fetal and parent blood vessels.
In the histological structure descripstion on the HE staining of Sunda porcupine placenta in section A1.3, the trophospongium area consist of cytotrophoblast and syncythiotrophoblast of the placental margin situated near to the maternal vessel and fetal vessel (Figure 5). In the histological structure description on the HE staining of Sunda porcupine placenta in section A1.4, there was villi and trophoblast cells in the fetal decidua basal zone (Figure 6). This section is included in the decidua basalis (Figure 7). According to Furukawa et al. (2011), the decidual cells that surround the blastocyte initially form a zone of primary decidua, which is avascular and dense with decidual cells. Primary decidua zones progressively degenerate, and placental and embryonic growth slowly replaces the secondary decidua zone, which is further reduced to a thin layer called decidua capsularis and decidua parietalis. Decidua mesometrial cells only form a thin layer at the base of the placenta called the decidua basalis, which is an important place for parent angiogenesis. The basal decidua is a developing blood vessel, which has an important role in the development of the interface of the decidua-vascularized placenta. Decidua can produce various hormones, cytokines, growth factors, and immunomodulatory molecules involved in the recruitment of limited but specific immune cell populations and placental growth (Roberts et al., 2016; Wheelhouse et al., 2016).
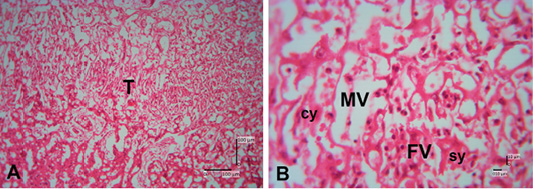
Figure 5: Photomicrograph of trophospongium area of Sunda porcupine placental disc (A1.3; HE). (A) 125x, and (B) 500x magnification. The trophospongium area at low (A) and high magnification (B). At (B) the trophospongium area consist of cytotrophoblast (cy) and syncythiotrophoblast (sy) of the placental margin situated near to the maternal vessel (MV) and fetal vessel (FV).
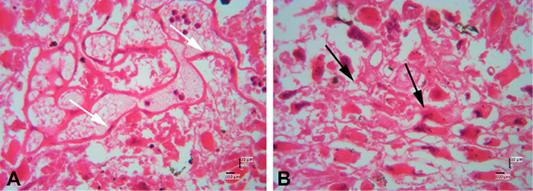
Figure 6: Photomicrograph of decidual area of Sunda porcupine placental disc (A1.4; HE). A and B are in high magnification (500 x). The white arrow shows the maternal epithelial cells that protrude to form villi. The black arrow shows trophoblast cells in the fetal part of the decidua zone.
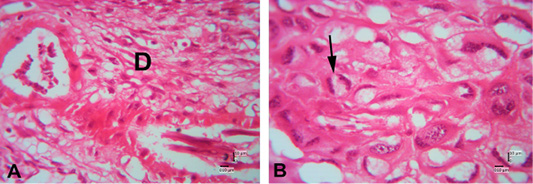
Figure 7: Photomicrograph of decidual area of Sunda porcupine placental disc (A1.5; HE). The decidua area at low (A) (125x) and high magnification (B) (500 x). At high magnification, decidua cells clearly seen (black arrow).
In the description of the histological structure of the Sunda porcupine placenta with HE staining there are several forms can be found in the basal region which are composed of three differentiated cell types: spongiotrophoblast, trophoblast giant cells and glycogen cells (Figure 8). Trophoblast cells are found with core one and nucleus two or called giant trophoblast cells.
The results of the HE staining on the Sunda porcupine placenta in section A1.5 show that the layers of this part are included in the metrial glands section. Metrial glands (Figure 9) are located in the mesometrial triangle from early pregnancy and fully develop in mid gestation, which leads to regression before birth or parturition. Metrial glands are composed of a dynamic mixture of cells from decidualized endometrial stromal cells, uterine killer cells (UNK), spinal-shaped arteries and fibroblasts (Furukawa et al., 2011; Walker et al., 2017). In the metrial glands section, there is spongiotrophoblasts are just above the trophoblast giant cell layer and glycogen cells form many masses of small cells and develop into glycogen cell islands in the middle of the period. According to Furukawa et al. (2014) spongiotrophoblasts are just above the trophoblast giant cell layer located at the interface of the materno-fetal placenta. Glycogen cells form many masses of small cells and develop into glycogen cell islands in the middle of the period, and then most of these glycogen cells disappear before or parturition.
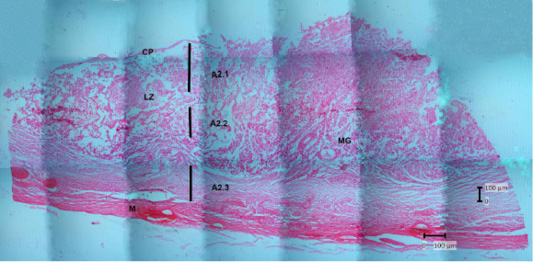
Figure 8: Photomicrograph of the thin parts of Sunda porcupine placental disc refers to Figure 1. A2 (HE). A2.1 is the deepest part, and A2.3 is the outer part. chorioallantoic plate (CP), labyrinth zone (LZ), metrial glands (MG), myometrium (M).
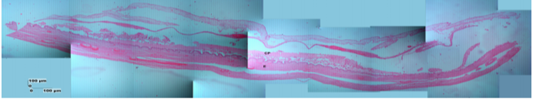
Figure 9: Photomicrograph of the thinest parts of Sunda porcupine placental disc refers to Figure 1. A3 (HE). chorioallantoic plate (CP), endometrium-myometrium layers (E).
The description of Sunda porcupine placenta with HE staining in thin samples (A3) shows a difference with samples A2 and A1, namely in the A3 sample, the endometrium and myometrium are still seen. According to Alimi et al. (2018), the endometrial section shows ciliated columnar epithelium and basal layer of lamina propria. Lamina propria is composed of loose connective tissue, collagen fibers, fibroblasts and lymphocytes that were found between tissues. The myometrium consists of smooth muscle, which is circularly arranged inside and longitudinal on the outside.
CONCLUSION
The results of this study indicate a similarity between placental Sunda porcupines and placenta Rodentia in general. In Sunda porcupines, the placenta has a flat shape like a disc. This type includes discoid type, which is a flat-shaped type such as a circular disc and the interaction is limited to a coarse circular area. Its histological structure is included in hemochorial, which is the most invasive type of placenta, and there is a direct relationship between the chorion and the parent blood. These results can be seen from the existing of villi that formed form the maternal (decidual) part, which formerly was maternal endometrial tissues, to fetal (labyrinth) and the trophoblast cells part of the fetus from the labyrinthine zone to the decidual basal zone. Thus, it can be said that the Sunda porcupine placenta is of type and structure is hemochorial.
ACKNOWLEGMENTS
This study was fully supported by the Directorate General of Higher Education (DIKTI), Ministry of Research, Technology and Higher Education of Indonesia, with contract number 38/LPPM UGM/2015.
Authors Contribution
Teguh Budipitojo developed the concepts and designed of the study, read and approved the final manuscript. Siti Shofiyah stained the tissue sample with Hematoxylin-Eosin staining method, data analysed and interpreted the data. Dian Bekti Hadi Masithoh wrote the manuscript, data analysed and interpreted the data. Linda Miftakhul Khasanah processed sample tissues for paraffin-embedded method and wrote the manuscript. Irma Padeta carried out euthanized the Sunda porcupines, collected samples and fixed samples in Bouin’s solution, read and approved the final manuscript.
Conflict of interest
All the authors declare that there is no competing interest in this study.
REFERENCES






