Advances in Animal and Veterinary Sciences
Research Article
Clinical and Gross Pathological Investigations of Internal Laying in Commercial Layer Chicken
Palani Srinivasan1, Gurusamipalayam Amirthalingam Balasubramaniam1, Thippichettipalayam Ramasamy Gopala Krishna Murthy2, Perumal Balachandran1
1Department of Veterinary Pathology, Veterinary College and Research Institute, Namakkal, India; 2Poultry Disease Diagnosis and Surveillance Laboratory, Veterinary College and Research Institute Campus, Namakkal, India.
Abstract | Avian oviduct is a tubular organ and its muscular contraction responsible for recurring ovum transportation, egg formation, oviposition, sperm transport and fertility. Any defect in oviduct motility may have a great bearing on the above said functions and also results in internal laying or ectopic oviposition. Modern commercial layer are highly prolific and susceptible to internal laying however their prevalence, nature and significance is not known. Hence a study was undertaken to assess the prevalence and clinico-pathological aspects of internal laying in commercial white leghorn layer chicken in Namakkal region of India. A total of 5145 carcasses of white leghorn layers, above 20 wks age from 255 flocks were examined for various oviduct abnormalities for a period of four years (2006 to 2009). Among all the cases examined for reproductive tract abnormalities, 51 (0.99%) cases from nine flocks revealed internal laying with an overall drop in egg production, morbidity and mortality of 0.5 to 1.0%, 0.5 to 1.0% and 0.2 to 0.5 % respectively between 21 and 80 wks. of age. Necropsy examination revealed distended abdomen, few to numerous, partial as well as fully formed eggs along with yolk materials in the peritoneal cavity. On the basis of oviduct lesions, the causes of internal laying were identified as thin shelled eggs (33.33%), salpingitis (27.45%), vent trauma (17.65), heat stress (3.92%), oviduct neoplasm (1.96%) and unknown cause (15.69%). Microbial analysis of samples revealed the presence of Escherichia coli in cases associated with salpingoperitonitis. Internal laying was noticed in layers with highest occurrence in 21–30 wk. (25.49%) and 41-50 wk. (21.57%) of age and during summer season (49.02%). It was concluded that the presence of eggs in the abdominal cavity and lesions in the shell gland and vagina indicates the functional alterations of the distal parts of reproductive tract could be responsible for internal laying.
Keywords | Internal layer, Prevalence, Gross pathology, Layer chicken, Oviduct, Etiology, Clinical Signs
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | November 24, 2014; Revised | December 27, 2014; Accepted | December 29, 2014; Published | January 02, 2015
*Correspondence | Palani Srinivasan, Veterinary College and Research Institute Campus, Namakkal, , India; Email: srinipat2004@yahoo.com
Citation | Srinivasan P, Balasubramaniam GA, Murthy TRGK, Balachandran P (2015). Clinical and gross pathological investigations of internal laying in commercial layer chicken. Adv. Anim. Vet. Sci. 3(1): 71-78.
DOI | http://dx.doi.org/10.14737/journal.aavs/2015/3.1.71.78
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2015 Srinivasan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The avian oviduct is a highly convoluted muscular tube responsible for forming the eggs by secretion of components surrounding the yolk. It extends from the single ovary to the cloaca and occupying a large part of the abdominal cavity. The development of modern strains of layer chicken that have been genetically selected for productivity traits will ovulate more in order to produce more number of eggs during laying period. Such enhanced genetic potential along with the sophisticated feeding management strategies make the bird susceptible to different types of reproductive disorders. Although it is well known that reproductive disease of poultry results in significant economic loss by decreased egg production and increased mortality, avian reproductive pathology is still in its infancy compared to mammalian reproductive disorders (Solomon, 2002; Crosta et al., 2003).
The sequence of events leading to oviposition is well documented and begins with the release of the mature ovum into the fimbriated funnel-shaped end of the oviduct (the infundibulum). Muscular contraction encourages the yolk mass to move distally through the neck of the infundibulum and thereafter into the magnum (Aitken, 1971; Solomon, 1991). The term “false layer” is used to describe a bird which exhibits normal production characteristics, but which at necropsy reveals a malfunctioning infundibulum. Under these circumstances the body cavity is filled with liquid or coagulated yolk. Birds with soft-shelled and fully formed eggs in the peritoneal cavity is called internal layer or ectopic egger and it occurs due to reverse peristalsis of oviduct (Srinivasan et al., 2014 a). Presence of ectopic eggs in abdominal cavity was commonly noticed in companion birds and in one study it was the most commonly (28.6%) observed reproductive disorder (Keymer, 1980).
Modern commercial layers are highly prolific and susceptible to internal laying, however very little is known regarding this condition (Batra and Singh, 1978; Srinivasan et al., 2014a) and their etiological factors were not investigated. To ensure persistent and maximum production in poultry flocks, it is imperative to investigate the internal laying of chicken in order to understand its prevalence, nature and significance of this disorder. Therefore the present study was planned to determine the prevalence and clinico-pathological aspects of internal laying in commercial layer chickens in Namakkal poultry zone of India.
Materials and Methods
Flock History
The study was carried out over a period of four year (2006 to 2009). A total of 5145 carcasses of white leghorn layers with oviduct abnormalities, above 20 wk. of age belonging to 255 commercial poultry flocks situated in and around Namakkal district of Tamil Nadu State, India were examined for the prevalence of internal laying. The flocks showing internal laying were inspected and collected information regarding shed capacity, strain of chicken, flock size, cage size, management and hygiene, feed additives, vitamins- mineral supplements and treatment given and egg production traits. To study the seasonal variations in the incidence of the internal laying, the whole year was divided into four seasons namely summer (March, April and May), south west monsoon (June, July and August), north east monsoon (September, October and November) and winter (December, January and February). According to the age, layers were grouped as 21 – 30 wk. 31- 40 wk. 41 – 50 wk. 51 – 60 wk. 61 – 70 wk. and 71 – 80 wk.
Pathological Examination
The dead birds were surface disinfected and necropsies were performed as per approved procedure (Chauhan and Roy, 2007). The area of the vent and cloaca was examined for signs of inflammation and traumatic injury. The carcasses were thoroughly examined for gross pathological changes including presence of eggs and its physical appearance, size and number, inflammatory signs and exudates in the peritoneal cavity, ovary, oviduct or all of them. Oviduct from affected birds were removed and opened along its longitudinal axis for examination of lumen and mucosal surface for the presence of yolk, albumin, shell or shell membrane, inflammatory exudate, malformation and obstruction.
Isolation of Causative Agent
Heart blood, liver and oviduct swabs were collected from 51 carcasses which shown the lesion of internal laying for screening of bacterial agents. The samples were placed in Brain Heart Infusion (BHI) broth and incubated at 37°C for 24 h and cultured aerobically in Brian heart infusion agar (BHIA), MacConkey’s agar and eosin methylene blue agar (EMBA) for isolation of bacteria. Bacterial isolates were identified on the basis of their morphology, growth characteristics, sugar fermentation and biochemical characteristics (Quinn et al., 2011). Pooled organ samples (trachea, lung, spleen, caecal tonsil, kidney and oviduct) collected from internal laying cases were subjected to haemagglutination (HA) test for detection of NDV (Alexander and Senne, 2008; Mohammad et al., 2013), IBV (Villarreal, 2010) and EDS-76 virus (Alam et al., 2009). Serum samples collected randomly from ten birds from flocks affected with internal laying were examined by haemagglutination inhibition (HI) test for the presence of antibodies to NDV, IBV and EDS virus and by ELISA for the Mycoplasma gallisepticum (Mg) and Mycoplasma synoviae (Ms).
Table 1: Age and season wise distribution and proportionate incidence of internal laying in commercial layer chicken.
|
S. No |
Cause of internal laying |
Age (wks) |
Season |
No. of cases |
Proportionate incidence (%) encountered |
||||||||
|
21- 30 |
31-40 |
41- 50 |
51-60 |
61-70 |
71-80 |
Summer SWM |
NEM Winter |
||||||
|
1. |
Thin shell egg |
04 |
03 |
04 |
03 |
02 |
01 |
13 |
03 |
-- |
01 |
17 |
33.33 |
|
2. |
Salpingitis |
02 |
02 |
04 |
03 |
02 |
01 |
01 |
06 |
05 |
02 |
14 |
27.45 |
|
3. |
Vent trauma |
02 |
03 |
02 |
01 |
01 |
-- |
05 |
02 |
01 |
01 |
09 |
17.65 |
|
4. |
Heat stress |
02 |
-- |
-- |
-- |
-- |
-- |
02 |
-- |
-- |
-- |
02 |
03.92 |
|
5. |
Oviduct neoplasm |
-- |
-- |
-- |
-- |
-- |
01 |
-- |
-- |
-- |
01 |
01 |
01.96 |
|
6. |
Unknown cause |
03 |
02 |
01 |
02 |
-- |
-- |
04 |
01 |
02 |
01 |
08 |
15.69 |
|
Total |
13 |
10 |
11 |
09 |
05 |
03 |
25 |
12 |
08 |
06 |
51 |
100.00 |
|
|
Percent |
25.49 |
19.61 |
21.57 |
17.65 |
09.80 |
05.88 |
49.02 |
23.53 |
15.69 |
11.76 |
|||
SWM- South west monsoon, NEM- North east monsoon
Results
Internal layer was diagnosed based on the presence of partially or fully formed egg in the peritoneal cavity on necropsy examination and laboratory investigation. The condition was recorded in 51 (0.99%) out of 5,145 birds examined over a period of four years (2006 to 2009). Birds affected with internal laying showed shrunken and cyanotic comb, depression, anorexia, distended abdomen, penguin like stance and pasting of vent region with yellowish faecal materials.
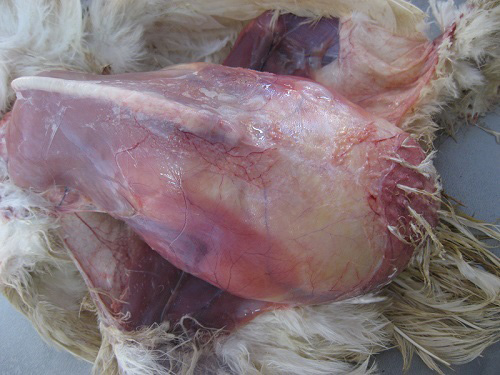
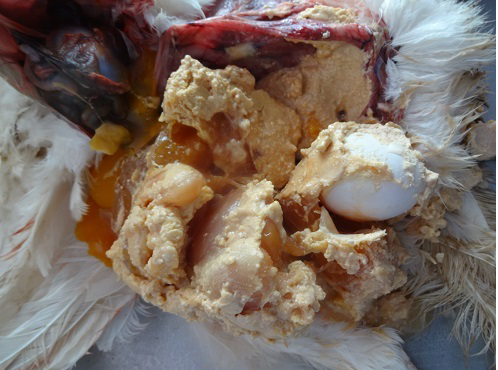
On necropsy examination, the condition of the carcass was fair to poor with prominent keel bone and distended abdomen (Figure 1). Peritoneal cavity contained liquid and coagulated yolk deposits and various sizes of few to numerous partial as well as fully formed eggs (Figure 2). The yolk materials coated the peritoneum, mesenteries and air sac membranes and caused yellowish discolouration and adherence of abdominal viscera with one another. In chronic cases peritoneum and abdominal organs showed blackish discolouration. Partially formed eggs with shell membrane alone were misshapen due to partial or complete loss of its contents and often only empty shell membranes were noticed in the peritoneal cavity. Fully formed eggs had multiple layers of albumin over the shell. Ovarian follicles exhibited normal appearance and hierarchy as well as partial to complete atretic changes associated with intrafollicular haemorrhages. Oviduct showed congestion of serosal blood vessels and mild to moderate reduction in size.

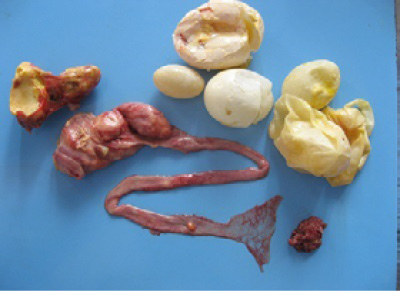
Figure 3: Oviduct lumen showing the partially formed egg, blood tinged albumin and yolk material and shell membrane.
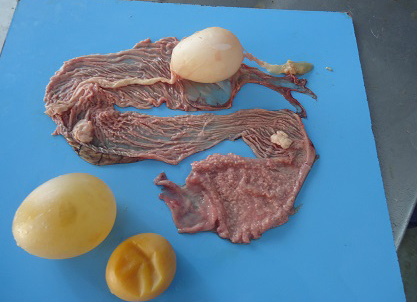
Figure 4: Oviduct lumen containing inflammatory exudate coated egg and wall showing inflammatory changes in salpingitis.
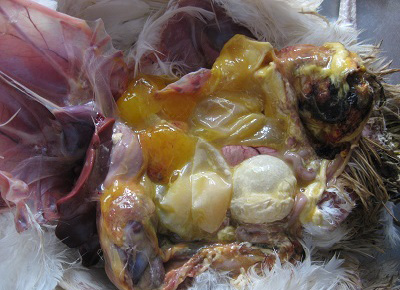
Figure 5: Cloacal region showing blackish discoloration and thin shelled eggs in peritoneal cavity in vent trauma.
Different causes of internal laying was determined based on the presence of characteristic lesions in the oviduct as shown in the table 1. In thin shell egg as sociated internal laying, oviduct lumen contained partially formed egg, shell membranes and admixture of yolk and albuminous materials with blood tinged appearance (Figure 3). Eggs that is moving towards infundibulum showed coating of albuminous materials which appeared as a concentric layers. Remnants of shell membranes were frequently noticed in the shell gland region of the oviduct. Birds with salpingitis induced internal laying, the oviduct showed partial or fully formed eggs coated with inflammatory exudate, congestion and mild to moderate thickening of the uterine wall along with the presence of eggs in peritoneal cavity (Figure 4). In traumatic injury of cloaca the vent region was thickened with blackish crusty materials and the peritoneal cavity contained thin shelled eggs and yolk materials (Figure 5). In heat stress affected birds uterus or peritoneal cavity contained thin shelled eggs and congestion of visceral organs. Internal laying associated with oviduct neoplasms revealed marked thickening of uterine and vaginal mucosa (Figure 6). In some birds, ectopic eggs were noticed without any specific lesions in visceral and reproductive organs.
Bacteriological examination of liver, heart blood and oviduct swabs collected from the 51 birds with internal laying and salpingo peritonitis lesions revealed the presence of E. coli. The organisms were identified based on lactose fermenting, pink colour round, smooth and glistening colonies on Mac Conkey’s agar, black colonies with metallic sheen on EMB agar, indole production at 44ºC, gas production in Eijkmann test and acid and gas production in differential sugar fermentation tests. Tissue samples collected for virological examination was found to be negative in haemagglutination test against NDV, IBV and EDS-76 virus. Serum samples collected from the affected flocks showed HI titre value ranging from 128 to 512 against NDV and IBV. The HI titre for EDS-76 and ELISA value for Mg and Ms were found to be negative.
Age and season wise occurrence of internal laying in commercial layer chicken was presented in table 1. Age wise analysis on the overall occurrence of internal layer in 51 commercial layer chicken showed highest occurrence in 21 to 30 wk. followed by 41-50 and 31–40 wk. age groups. Analysis of data on the season wise occurrence showed highest incidence during summer (49.02%) followed by south west monsoon, north east monsoon and winter season. Egg production drop, morbidity and mortality in the affected flocks were 0.5 to 1%, 0.5 to 1% and 0.2 to 0.5% respectively.
Discussion
Avian oviduct is responsible for the recurring ovum transportation, egg formation, oviposition, sperm transport and fertility. Any defect in oviduct motility will affect above said function and sometimes results in reverse peristalsis leading to ectopic oviposition or internal layer. Internal ovulation and internal layer are used interchangeably in domestic hen (Singh et al., 2013), however difference exists between these two terms. Internal ovulation means presence of yolk or yolk materials in the peritoneal cavity due to the unsynchronized action of ovary and oviduct resulting in ectopic ovulation where as in internal layer partial and fully formed eggs present in the peritoneal cavity (Srinivasan et al., 2014a). In the present study 0.99% of the birds showed internal laying, however Keymer (1980) reported 28.6%. The difference in the occurrence of internal laying between these studies might be due to variation in the duration of the study, management, climatic condition and type of birds utilized for the study.
An ovum released from the ovary is received by the infundibulum and enters into the uterus (shellgland) approximately 4 to 5 h thereafter and it stays in the uterus for approximately 20 h to form the eggshell and is expelled from the uterus through the vagina by the contraction of the uterine smooth muscle (Gilbert, 1971). On necropsy examination, ovarian follicle showed normal appearance as well as atretic changes and the peritoneal cavity contained yolk materials in birds died with internal laying. The yolk material arises from the bursting atresia of large ovarian follicle and also from the failure of the infundibulum to engulf the ovulating ova resulting in yolk bursting in the abdominal cavity (Romagnano, 1996). In normal laying hen, the ectopic yolk in the body cavity flows along with interstitial fluids and passively absorbed by the lymphatic vessels and delivered directly into the plasma and the peritoneal macrophages remove the remaining lipid that trapped in the extracellular matrix (Cornax et al., 2013). However this process might be disrupted by the presence of shell membranes and hardened egg masses leads to accumulation of coagulated yolk in the peritoneal cavity in the present study. Yolk is a very irritating substance to the serosal surface and initiated inflammatory response in the affected birds (Srinivasan et al., 2013). Presence of partially or fully formed eggs in abdominal cavity indicates that the yolk progressed normally through the oviduct up to isthmus or shell gland and then reverse peristalsis movement might discharged the eggs into the body cavity instead of progressing down the oviduct and being laid. The direction of transport could be easily changed by obstruction in oviduct, cystic hyperplasia, neoplasia, salpingitis, malnutrition, trauma and stress (Romagnano, 1996; Joyner, 1999; Johnson, 2000).
In the present investigation, 33.33% of dead birds with internal laying contained egg shell membranes in uterus and thin shelled eggs along with a fully formed eggs in the peritoneal cavity. This might be due to the prevalence of various stresses and nutritional imbalances during the early and peak egg production periods (Srinivasan et al., 2012) which impairs the formation of shell membrane mammillary layer (Solomon, 1991) and disorganization of subsequent egg shell layer leads to thin or soft shelled eggs. Thin shelled eggs are softer and more fragile than normal and it is very difficult for a bird to lay a soft-shelled egg. Acute stress elevate the plasma Arginine Vasotocin (AVT) level (Wang et al., 1989) and in turn increase the uterine motility, since the muscles that push the egg out tend to deform rather than moving it and sometime even rupture may also occurs leads to presence of thin shell membrane in the uterus (Srinivasan et al., 2014 b) which exerted its effects as a mechanical irritation to develop reverse peristalsis (Sturkie and Freedman, 1962). The oviductal smooth muscle is highly sensitive to mechanical irritation due to dense innervations of sympathetic and parasympathetic nerves (Gandahi et al., 2013).
In this field study, 27.45% of internal layer showed salpingitis which might cause reverse peristalsis through the narrowing of oviduct lumen by inflammatory reaction and increased production of prostaglandins (Heinonen, 1978). Prostoglandins are potent stimulants of most smooth muscles including the uterine portion of the oviduct through the effects of increasing the amount of AVT receptors in the uterus which induce abnormal oviduct motility in chicken (Hertelendy et al., 1974). Injuries on the vent (17.65% of birds) lead to inflammation and pain which will hinder egg laying (Kaikabo et al., 2007) and induce reverse peristalsis. Birds affected with heat stress (3.92 %) revealed the presence of thin shelled or shell less egg in uterus or peritoneal cavity. Laying hens exposed to high temperature, the plasma calcium level significantly reduced due to reduction in calcium use and uptake by uterine and duodenal epithelial cells respectively leads to hypocalcemia (Mahmoud et al., 1996). Oviduct neoplasm affecting the uterus and vagina (1.96 % of birds) caused narrowing of oviduct lumen resulting in mechanical obstruction of egg movement and induce the reverse peristalsis leading to internal oviposition (Bwala et al., 2011; Srinivasan et al., 2014b). Internal laying was noticed without any specific cause in 15.69% cases in the present investigation which was in agreement with Rosskopf and Woerpel (1987) they also reported that it may occur during normal laying process.
In the current investigation, E. coli organisms were isolated from all the dead birds with internal laying and salpingo peritonitis. Among the various bacteria that cause primary or secondary reproductive tract infections E. coli is most common in commercial layer chicken (Srinivasan et al., 2013). E. coli is a normal inhabitant of the chicken intestinal tract with up to 106 of these bacteria per gram of intestinal contents. Approximately 10 to 15 % of intestinal E. coli is considered to be potential pathogens. As long as the intestinal mucosal barrier is intact, the normal microflora of bird is likely to inhibit the translocation of pathogenic E. coli from the intestine to the blood stream and organs. When these barriers are damaged, possibly by the stress of lay and presence of yolk materials in the abdominal cavity might facilitate the pathogenic E. coli to invade and cause salpingo peritonitis (Leitner and Heller, 1992).
Though internal laying in chicken was recorded in all the four seasons, higher incidence was observed in summer compared to other seasons due to the high prevalence of nutritional imbalance and stress (Srinivasan et al., 2014b). Flocks with internal laying problem showed 0.5 to 1% drop in egg production. WoodGush and Gilbert (1970) reported 11.6 per cent egg loss due internal laying. Wide variation between these studies might be due to criteria used for evaluating the internal laying. In affected flocks, birds showed morbidity of 0.5 to 1 % and symptoms like depression, penguin like posture and pasting of vent region. Penguin-like posture observed in the present investigation might be due to progressive accumulation yolk material, partial and fully formed eggs along with inflammatory exudate in the abdominal cavity. In the present investigation, flocks affected with internal laying showed a mortality of 0.2 to 0.5 per cent. Mortalities from reproductive pathologies are rare and in most cases caused by other complications (Jordan et al., 2005).
Conclusion
Among the oviduct abnormalities of commercial layer chicken internal laying contributed 0.99 %. The disorder predominantly noticed in layers during peak egg production period (21 to 50 wk.) and summer season. It was observed that drop in egg production, morbidity and mortality in the flocks of current study were 0.5 to 1%, 0.5 to 1% and 0.2 to 0.5% respectively. The study provides valuable information on the prevalence and clinco-pathological aspects of internal laying in commercial layer chicken and will be useful for the researchers and veterinarians to develop strategies for both treatment and control of this problem in layer industry especially during the peak laying period.
Acknowledgements
The financial support and facilities provided by the Tamil Nadu Veterinary and Animal Sciences University, Chennai, India are duly acknowledged.
References