Advances in Animal and Veterinary Sciences
Spirulina platensis Alleviates Arsenic-Induced Toxicity in Male Rats: Biochemical, Histopathological and Immunohistochemical Studies
Kawkab A. Ahmed1, Reda M. S. Korany1*, Hanaa A. El Halawany2, Khaled S. Ahmed2
1Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; 2Department of Pathology and Reproductive Diseases, Animal Reproduction Research Institute (A.R.R.I.), Giza.
Abstract | This study was conducted to investigate the protective impact of Spirulina platensis (Sp) in sodium arsenate (As) induced toxicity in male rats. Twenty male Wistar albino rats were used and divided into four equal groups, 5 rats in each group, control negative group and three treatment groups that daily received Sp (300 mg/ kg body weight(bwt), As(5 mg/kg bwt), and Sp +As (300 mg/ kg bwt (Sp) + 5 mg/kg bwt (As)) respectively, for 2 months by oral gavages. Results showed that the body weight was significantly reduced in As-treated group compared to the control, while the co-treatment with Sp significantly recovered the body weight. Arsenic caused a significant reduction in the level of testosterone. Moreover, biochemical analysis revealed that arsenic significantly increased serum malondialdehyde (MDA), aspartate aminotransferase (AST), alanine aminotransferase (ALT) as well as creatinine and significantly reduced serum glutathione (GSH) level. Sp co-treatment significantly recovered the serum testosterone and GSH levels and significantly reduced MDA, AST, ALT and creatinine. Histologically, testes, liver and kidneys of arsenic-treated group showed different histopathological alterations associated with strong positive caspase-3 immune reaction. Spirulina co-treatment alleviated the histopathological changes with weak expression of caspase-3 protein. We could conclude that Spirulina platensis successfully mitigated the abovementioned parameters induced by arsenic toxicity in different organs of male rats.
Keywords | Arsenic, Spirulina platensis, Oxidative stress, Histopathology, Immunohistochemistry, Male rats.
Received | Janiary 21, 2019; Accepted | May 14, 2019; Published | June 30, 2019
*Correspondence | Reda M.S. Korany, Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, 12211, Egypt; Email: reda.pathology@yahoo.com
Citation | Ahmed KA, Korany RMS, El Halawany HA, Ahmed KS (2019). Spirulina platensis alleviates arsenic-induced toxicity in male rats: biochemical, histopathological and immunohistochemical studies. Adv. Anim. Vet. Sci. 7(8): 701-710.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.8.701.710
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Ahmed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Arsenic is currently one of the most important environmental global contaminants and toxicants, particularly in the developing countries. The main route of arsenic exposure in these countries is drinking water; some rocks of earth’s crust contain this element and the use of herbicides and insecticides increases the concentration of arsenic in ground water (Garcia et al., 2013).
Arsenic exposure occurs from inhalation, absorption through the skin and primarily, by ingestion of contaminated food and drinking water. As-induced toxicity depends on its oxidation state and chemical form. Inorganic form of As is more toxic than organic form (Manna et al., 2008).
Arsenic exposure causes obvious damage in various organs. Total tissue arsenic accumulation was greatest in kidney, lung, urinary bladder, skin, blood and liver (Kenyon et al., 2008). Chronic exposure to inorganic arsenic has been associated with loss of body weight, cancer of the skin, liver fibrosis, and chronic lung disease. Moreover, it causes metabolic disorders such as diabetes and dysfunction of the endocrine system, nervous system, cardiovascular diseases and reproductive system (Elshawarby et al., 2014; Ma et al., 2015). Arsenic binds to sulfhydryl groups on proteins, modulates protein metabolism, and generates reactive oxygen species (ROS) (Mehta and Hundal, 2016). The free radical-mediated theory becomes more acceptable to explain the damage induced by arsenic. Oxidative stress is acceptable as one of the mechanisms of arsenicosis, which can be mediated by excess production of reactive oxygen species (ROS) (li et al., 2015). For that reason, nutritional antioxidants in diseases related to oxidative stress have gained immense interest in recent years because they could be used as suitable preventive / therapeutic agents (Pineda et al., 2013).
Amelioration of arsenic toxicity has been investigated by many researchers using many agents, natural and artificial. One of these agents used recently as a potential ameliorating agent against arsenic toxicity is Spirulina platensis (Sp) (Islam et al., 2014; Sayed et al., 2015; Bashandy et al., 2016; Hasan et al., 2015).
Spirulina platensis, also known as Arthrospira platensis, is a filamentous blue-green microalga (cyanobacterium) (Ouhtit et al., 2014) that grows in alkaline lakes, has a high protein content and has been used as animal feed (Ayehunie et al., 1998).
In recent years, Spirulina has attracted more attention as a potential source of pharmaceutical compounds. Many researches showed that Spirulina has numerous health benefits, including antioxidant, immunomodulatory, anti-inflammatory, anticancer, anti-viral, and anti-bacterial activities, as well as positive effects against hyperlipidemia, malnutrition, obesity, diabetes, heavy metal chemical-induced toxicity, and anemia (Wu et al., 2016).
Aim of Work
Assessment of the protective potential of Spirulina platensis against arsenic toxicity in male rats.
Chemicals
All chemicals used in this study were of highest available purity. Pure premium spirulina powder was obtained from Arab Academy for Science, Technology and Maritime Transportation, Alexandria, Egypt. Sodium arsenate (Na3AsO4) obtained from the representative of Fisons Scientific Apparatus Ltd. UK in Egypt. All kits for biochemical analysis were purchased from Biodiagnostics, Egypt.
Experimental Animals
Adult male Wistar albino rats weighing (120-150 gm) used in this experiment were obtained from Helwan Animal Colony belonging to VACSERA. Rats were housed in standard cages kept in a ventilated room under controlled laboratory conditions of normal light-dark cycle (12 hours light/dark) and temperature (25 ± 2˚C). Standard laboratory chow and water were provided ad libitum. Animals allowed acclimatizing for a week before commencement of the study. The experimental protocol was approved by the institutional Animal care and Use Committee (CUIACUC), Cairo University, Egypt (approval No. CU-II-F-37-18).
Experimental Design
Twenty adult male Wistar albino rats were randomly allocated into four groups (n=5 rats).Group 1 served as normal control group received normal saline, group 2 daily administered Sp (300 mg/ kg bwt) dissolved in water by oral gavage, group 3orally gavages by sodium arsenate (5 mg/kg bwt) dissolved in normal saline and group 4was co-administered with Sp (300 mg/kg body weight) and sodium arsenate (5 mg/kg body weight) (Bashandy et al., 2016). The animals body weight was measured after one month and two months.
Sampling
At the end of the experimental period (2 months), rats were euthanized 24 hours after last treatment and blood samples were collected from the retro-orbital venous plexus (Sorg and Buckner, 1964) in sterile centrifuge tubes and left to clot and centrifuged at 3000 rpm for 15 minutes to separate sera. Sera aliquots were stored at -20˚C for further biochemical analysis. Testes, liver and kidneys from each rat were immediately removed and fixed in neutral buffer formalin for histopathological and immunohistochemical examination.
Estimation of Testosterone
Testosterone hormone in sera was determined by EnzymeImmunoassay Method (ELISA), Biocheck, Inc, 323 vintage Park Dr. Forster City, CA, USA, according to the kit manufacture instructions.
Evaluation of Oxidative Stress Markers
Lipid peroxidation was evaluated by measurement of serum MDA (Ohkawa et al., 1979) based on the formation of thiobarbituric acid reactive substances (TBARs) and expressed as the extent of malondialdehyde (MDA) production. The non-enzymatic antioxidant biomarker, GSH was assessed (Beutler et al., 1963).
Evaluation of Biochemical Parameters
Liver alanine aminotransferase (ALT or GPT), aspartate aminotransferase (AST or GOT) activities were measured (Reitman and Frankel, 1957) and Kidney creatinine levels were measured calorimetrically (Schirmeister et al., 1964) by the commercially available kits from Biodiagnostics, Egypt.
Histopathological Examination
Specimens from testes, liver and kidneys were collected and fixed in neutral buffered formalin 10%, washed, dehydrated, cleared and embedded in paraffin. The paraffin embedded blocks were sectioned at 4-5 micron thickness and stained with Hematoxylin and Eosin (Bancroft et al., 2012) for histopathological examination by a light microscope (Olympus BX50, Japan).
Grading of Histopathological Alterations
Histopathological alterations were graded as (0) indicated no changes, (1), (2) and (3) indicated mild, moderate and severe changes, respectively, while the grading was determined by percentage as follows: (<30%) showed mild changes, (<30% – 50%) indicated moderate changes and changes more than 50% (>50%) indicated severe changes (Arsad et al., 2014).
Immunohistochemistry
Formalin-fixed, paraffin-embedded 4 µm sections were fixed into poly-L-lysine coated slides (Thermo-Scientific, Karlsruhe, Germany). After deparaffinization and rehydration, the slides were immersed in buffer Target Retrieval Solution, pH 9.0 (Dako, Glostrup, Denmark). Peroxidase Blocking Solution (Dako, Glostrup, Denmark) was used to block activity of endogenous peroxidase. The slides were incubated with 1 mg/ml of caspase-3 antibodies at 1:50 dilution (DAKO, Glostrup, Denmark) and immunostained with DAKO RealTM EnvisionTM Detection System Peroxidase/DAB+, HRP Rabbit/Mouse (Dako, Glostrup, Denmark). The positive immune reactive cells showed brown-stained cytoplasm. Staining intensity and its distribution were graded as negative (no staining), weak, moderate, or strong intensity. Quantification of caspase-3apoptotic protein was estimated by measuring the area % expression from 5 randomly chosen fields in each section and averaged using image analysis software (Image J, version 1.46a, NIH, Bethesda, MD, USA).
Statistical Analysis
All results were expressed as mean ± SD (Standard Deviation). Statistical analyses were performed using the SPSS version 24.0 statistical analysis package (SPSS, Inc., Chicago, IL, USA). The parametric test one-way ANOVA was used for data analysis and comparison was done using Turkey post-hoc test. In all calculations, a difference at P < 0.05 or P<0.001 was considered significant.
Results
Body Weight
The result of the animals body weight was presented in Figure 1.The As-treated group showed significant reduction in the rats body weights (P < 0.01 and p < 0.001) in comparison with the control group, while co-treatment with Spirulina significantly recovered the body weight in comparison with As-treated group as seen during the period of experiment (1 and 2 months).
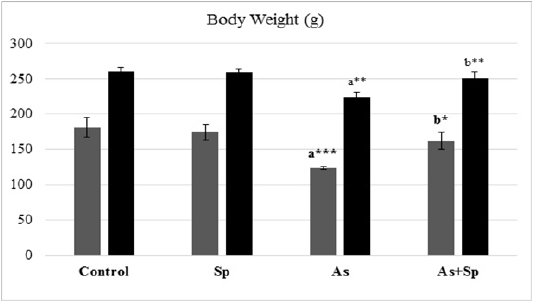
Figure 1: Effect of arsenic and spirulina platensis on the body weight among different experimental groups. The values are expressed as the means ± SD, where n=5. Superscript a refers to a significance from control group, Superscript b refers to a significance from As group in the same column.
* refers to P< 0.05, ** refers to P< 0.01, *** refers to P< 0.001.
Sp: Spirulina; As: arsenic; As+Sp: Arsenic and Spirulina.
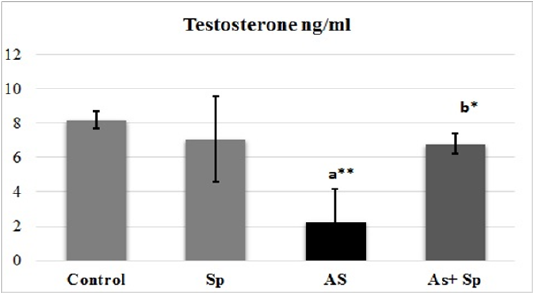
Serum Total Testosterone
The results of testosterone were presented in Figure 2. The levels of serum testosterone exhibited significant decrease (P< 0.01) in As-treated group in comparison with the control group. These levels showed a significance (P< 0.05) recovery towards control levels in rats co-administered spirulina with As (Sp +As treated group).
Superscript a refers to a significance from control group, Superscript b refers to a significance from As groups. * refers to P < 0.05, ** refers to P < 0.01. Sp: Spirulina; As: arsenic; As+Sp: Arsenic and Spirulina.
Serum Oxidative Stress Markers
Effects on serum GSH and MDA in different experimental groups were illustrated in Figure 3. The results revealed a significant depletion (P< 0.01 and P< 0.001) of serum GSH in the As-treated rats in comparison with control. While the co-administration with Spirulina platensis resulted in a significant increase (P< 0.01 and P< 0.001) in serum antioxidant capacity (GSH) in comparison with As-treated rats. On the other hand, serum MDA in As-treated rats showed a significant elevation (P< 0.01 and P< 0.001) in comparison with the controls. Meanwhile, the co-treatment with Spirulina platensis revealed a significant decrease of MDA levels in comparison with As-treated group (P< 0.01 and P< 0.001).
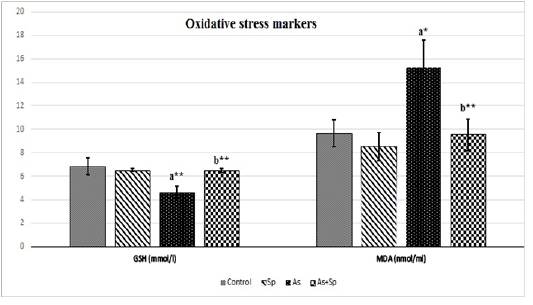
Figure 3: Effect of arsenic and spirulina platensis on serum GSH and MDA ± SD among different experimental groups (n=5) during the study. Superscript a refers to a significance from control group, Superscript b refers to a significance from As groups. * refers to P < 0.05, ** refers to P < 0.01. Sp: Spirulina; As: arsenic; As+Sp: Arsenic and Spirulina; GSH: Reduced glutathione; MDA: Malonaldehyde
Liver and Kidney Functions Parameters
The result of the levels of serum AST, serum ALT and serum creatinine was presented in Figure 4, where the serum AST, ALT and creatinine levels exhibited significant increase (P < 0.01 and P < 0.001) in As-treated animals when compared with the control one. These serum parameters showed a significant recovery (P < 0.01 and P < 0.001) when rats co-administered Spirulina with arsenic.
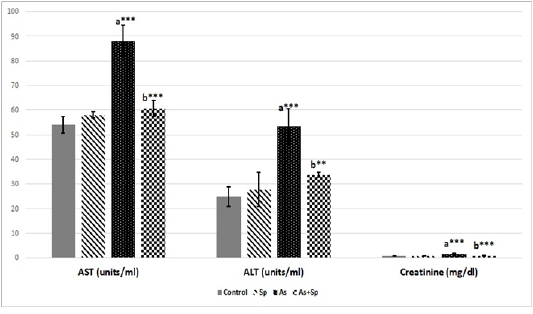
Figure 4: Effect of arsenic and spirulina platensis on serum ALT, AST and creatinine ± SD among different experimental groups (n=5) during the study. Superscript a refers to a significance from control group, Superscript b refers to a significance from As group. * refers to P < 0.05, ** refers to P < 0.01. Sp: Spirulina; As: arsenic; As+Sp: Arsenic and Spirulina; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Table 1: Grading of histopathological lesions in testes, liver and kidneys in all groups
| Organs | Lesions | Control | Spirulina | Arsenic | AS+SP |
| Testes | - Degeneration and necrosis of spermatogonial cells - Spermatid giant cells in the lumen of seminiferous tubules - Interstitial edema |
0 0 0 |
0 0 0 |
3 2 2 |
0 0 0 |
| Liver | -Congestion of central vein and hepatic sinusoids - Kupffer cell activation -Cytoplasmic vacuolization of hepatocytes -Focal necrosis of hepatocytes -Karyomegaly of hepatocytic nuclei -Portal fibrosis with massive mononuclear inflammatory cells infiltration |
0 0 0 0 0
0 |
0 0 0 0 0
0 |
2 3 3 2 3
3 |
1 1 1 0 1
1 |
| Kidneys | -Vacuolar degeneration of epithelial lining renal tubules - Congestion of renal blood vessels and glomerular tufts -Focal necrosis of epithelial lining renal tubules - Interstitial mononuclear inflammatory cells infiltration |
0 0 0 0 |
0 0 0 0 |
2 2 2 2 |
1 1 0 0 |
The score system was designed as: score 0 = absence of the lesion in all rats of the group (n= 5), score 1= (<30%), score 2= (<30% – 50%), score 3= (>50%).
Histopathology
Histopathological alterations of testes, liver and kidneys were graded and scored according to their severity as shown in Table (1). The scoring of lesions in different groups declared that co- treatment with Spirulina ameliorated the arsenic-induced pathological changes in the examined organs.
Microscopically, testes of control and Spirulina treated rats revealed the normal histological organization and architecture of seminiferous tubules with normal spermatogonial cells, complete spermatogenesis and sperm production (Figure 5a). In contrary, testes of As-treated group showed marked degeneration and necrosis of spermatogonial cells lining seminiferous tubules. The affected seminiferous tubules became void of cellularity with reduced diameter of seminiferous tubules and interstitial edema (Figure 5b). Degenerated tubules showed irregular outline of basement membrane as it sometimes was empty of any cellularity. Marked necrosis and exfoliation of germ cells with formation of spermatid giant cells in the lumen of some seminiferous tubules (Figure 5c) were also observed. Meanwhile, these alterations were less dramatic in Sp+As treated group. The examined sections showed an evidence of maintaining the normal seminiferous tubules with no histopathological alterations (Figure 5d). Concerning liver, examined sections from control as well as spirulina treated rats revealed the normal histological structure of hepatic lobules (Figure 6a). On the other hand, liver of arsenic treated rats revealed distorted hepatic structure, with dilatation and congestion of central vein and hepatic sinusoids. Kupffer cell activation and marked cytoplasmic vacuolization of hepatocytes were noticed in almost all examined sections (Figure 6b). Focal necrosis of hepatocytes associated with mononuclear cell infiltration as well as apoptosis of hepatocytes (Figure 6c) was also clearly noticed in many examined sections.Some examined sections revealed karyomegaly of hepatocytic nuclei. Portal areas showed portal fibrosis with formation of newly formed bile ductules and massive mononuclear inflammatory cells infiltration. Meanwhile, marked ameliorative effect was noticed in examined sections from rats co-treated with As+Sp. The examined sections from this group revealed mild histopathological alterations confined as slight hydropic degeneration of hepatocytes and slight congestion of hepatic sinusoids (Figure 6d).
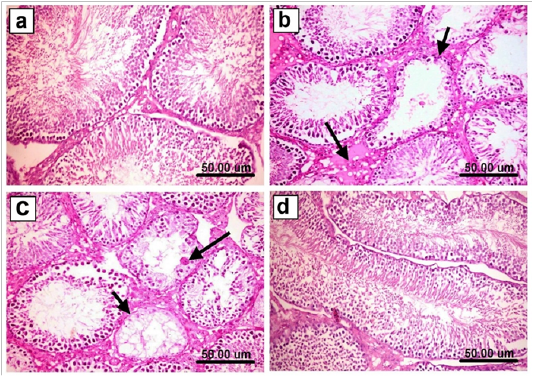
Figure 5: Testis of rat from a) control, untreated group showing the normal histological structure of seminiferous tubules with normal spermatogonial cells and complete spermatogenesis with sperm production, b) As-treated group showing marked degeneration and necrosis of spermatogonial cells lining seminiferous tubules, the affected seminiferous tubules became void of cellularity with reduced diameter of seminiferous tubules (short arrow) and interstitial edema (long arrow), c) As-treated group showingmarked necrosis, exfoliation and absence of germ cells (short arrow) with formation of spermatid giant cells in the lumen of some seminiferous tubules (long arrow), d)Sp+As co- treated group showing an evidence of maintaining the normal seminiferous tubules with no histopathological alterations and complete spermatogenesis.(H & E scale bar 50 um).
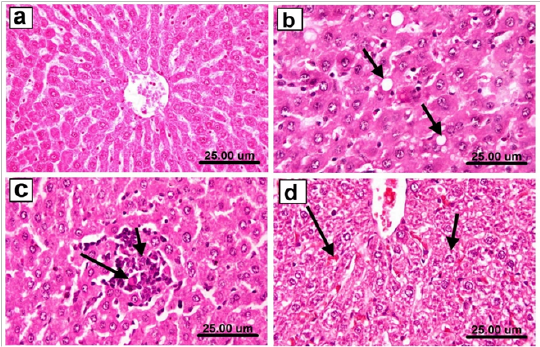
Figure 6: Liver of rat from a) control group showing the normal histological structure of hepatic lobules, b) arsenic treated group showing Kupffer cell activation and marked cytoplasmic vacuolization of hepatocytes (arrows), c) arsenic treated group showing focal necrosis of hepatocytes associated with mononuclear cell infiltration as well as apoptosis of hepatocytes, d) Sp+As co- treated group showing slight hydropic degeneration of hepatocytes (short arrow) and slight congestion of hepatic sinusoids (long arrow) (H & E scale bar 25 um).
Regarding kidneys, sections from control negative as well as Sp treated rats revealed the normal histological structure of renal parenchyma (Figure 7a). In contrary, kidneys of rats treated with arsenate showed different histopathological alterations described as vacuolar degeneration of epithelial lining renal tubules, congestion of renal blood vessels, focal necrosis of epithelial lining renal tubules associated with mononuclear cells infiltration as well as cystic dilatation of some renal tubules (Figure 7b). Moreover, some sections from this group revealed interstitial peritubular mononuclear inflammatory cells infiltration (Figure 7c) and some renal tubular epithelium showed karyocytomegally. On the other hand, kidneys of rats co-administered As+Sp revealed marked improvement of the previously mentioned lesions, most examined sections revealed mild histopathological alterations as slight vacuolar degeneration of epithelial lining some renal tubules and slight congestion of renal blood vessels and glomerular tufts. (Figure 7d).
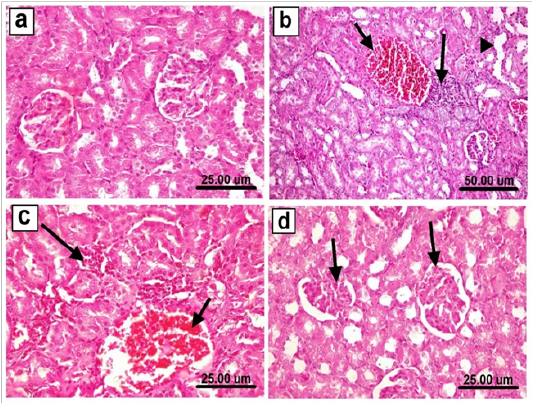
Figure 7: Kidneys of rat from a) control group showing the normal histological structure of renal parenchyma, b) arsenic treated group showing congestion of renal blood vessels (short arrow), focal necrosis of epithelial lining renal tubules associated with mononuclear cells infiltration (long arrow) as well as cystic dilatation of some renal tubules (arrow head), c) arsenic treated group showing congestion of renal blood vessel (short arrow) and interstitial peritubular mononuclear inflammatory cells infiltration (long arrow), d) As+Sp co-administered group showing slight congestion of glomerular tufts (arrows). (H & E scale bar 25 um (a, c & d) and 50 um (b)).
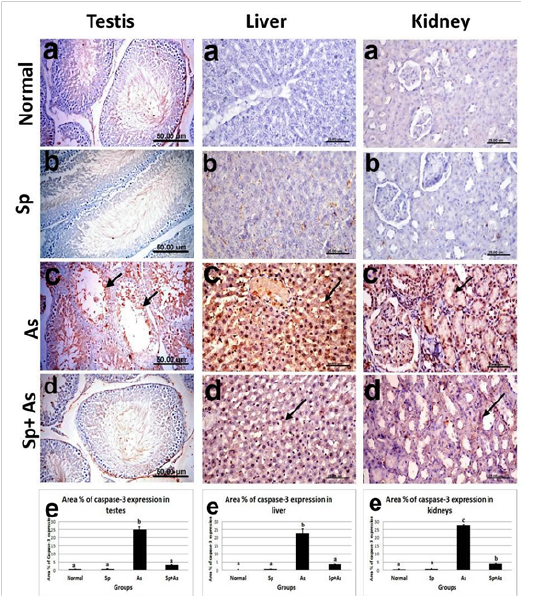
Figure 8: Immunostaining of caspase-3 in testes, liver and kidney of, a) control rat showing no caspase-3 immune-reactive cells, b) Sp- treated rat showing no caspase-3 immune-reactive cells. c) arsenic-treated rat showing strong positive immune expression. d) Sp + As co-treated group showing weak positive caspase-3 expression. e )immunostaining area % of caspase-3 expression. Data shown as mean±SE; error bars showing the variations of determinations in terms of standard error. One−way analysis of variance was used for data analysis (n=5), mean values with unlike superscript letters were significantly different (p ≤ 0.05).
Immunohistochemistry
Caspase-3 expression: Immunostaining of caspase-3 protein in testes, liver and kidneys sections revealed no caspase 3 immune-reactive cells in the testes, liver and kidneys of rats from control as well as spirulina treated groups (Figures 8a & b). Meanwhile, sections from arsenic treated rats showed strong positive immune-reactive cells (Figure 8c). On the other hand, co-administration of Spirulina (As+Sp group) revealed weak expression of caspase-3 protein in testicular, hepatic and renal tissues (Figure 8d). Figure 8e revealed the evaluation of immunostaining expression of caspase-3 % in the rats from different experimental groups.
Discussion
Arsenic-induced toxicity depends upon the forms of arsenic and the administration routes which greatly affect the severity of the toxicity (Wang and Holladay, 2006). Many studies, including assessed the effect of arsenic on male reproductive system, demonstrating its devastating effects on the male reproductive system. In male some studies demonstrated the toxic effect on spermatogenesis and hormonal profile (Ferreira et al., 2012; Li et al., 2012; Huang et al., 2016; Souza et al., 2016). Recently many studies have investigated the amelioration of As-induced toxicity using many antioxidants as scavengers for the free radicals and ROS generated by arsenic exposure (Chattopadhyay et al., 2001; Reddy et al., 2011; Garcia et al., 2013; Amer et al., 2016).
The adverse effect of arsenic on body weight has been proven previously in many reports on different animal species (Rodriguez et al., 2016; Elshawarby et al., 2014; Khan et al., 2014). Furthermore, in the present study, this adverse effect is significantly (P< 0.01 and P< 0.001) proven. As-treated rats showed a significant decrease in body weight in comparison to the control rats. Although (Chatterjee and Chatterji, 2010; Ferreira et al., 2012; Souza et al., 2016) reported that arsenic exposure did not affect the total body weight in male rats in their studies. This may be attributed either to the dose or duration of their studies. Noticeably, the significantly reduced or retarded body weight of As-treated rats was significantly recovered by Sp co-administration. This improving effect on the body weight in As+Sp group could be attributed to the Spirulina platensis unique blend of nutrients that no single source can offer, like B-complex vitamins, minerals, proteins, γ-linolenic acid and the super anti-oxidants such as β-carotene, vitamin E (Kulshreshtha et al., 2008; Holman and Malau-Aduli, 2012). It is well accepted that arsenic causes cellular toxicity by elevating oxidative stress and impairing antioxidant enzymes of the cells which in turn leads to toxicity of different cellular macromolecules and alteration in the signaling molecules, ultimately causing cellular death (Jomova et al., 2011).
Hormonal assay in this study indicated the significant decreased levels of testosterone in As-treated rats, this effect may be attributed to many aspects as the As-induced reproductive toxicity is responsible for the decrease in testes and accessory sex organ weights as well as decline in synthesis of male reproductive hormone especially testosterone. Testosterone is the major hormone that controls the process of spermatogenesis in testis (Zubair et al., 2016). The decrease in serum testosterone could be due to decreased responsiveness of Leydig cells to leutinizing hormone and/or the direct inhibition of testosterone steroidogenesis. Testosterone plays an important role in attachment of the germ cells in seminiferous tubules. Low levels of intra-testicular testosterone may lead to detachment of germ cells from seminiferous epithelium and may initiate cell apoptosis. The decreased levels of testosterone might affect the status of reproductive potential of these animals (Ali et al., 2013).
In our study, the biochemical analysis, exhibited a significant elevation of liver enzymes (AST and ALT), and these results were consistent with the previous studies (Flora et al.,1997; Sayed et al., 2015). The exact mechanism involved in elevation of these enzymes has not been conclusively postulated. Several workers have suggested that such effect may be the result of cellular damage or increased plasma membrane permeability. Moreover, factors such as increased synthesis or decreased enzyme degradation may also be involved (Sayed et al., 2015). On the other hand, in the present study, the co-administration of Sp significantly reversed the levels of AST, ALT to values near to control level. Additionally, this study showed that As-treatment result in significant increase of serum creatinine levels thereby indicating impaired renal function. These results were in collaboration with the previous findings of Amer et al. (2016); Zhang et al. (2014). This elevation of serum creatinin, has been shown to be significantly reversed by the Sp co-administration.
Histopathologically, the deleterious effects of arsenic on the testes, liver and kidneys were clearly evident in this study. The histopathological analysis of testes showed that arsenic has an adverse effect on the seminiferous tubules at the given dose (5mg/kg BW), consequently on the spermatogenesis process as a whole, as some seminiferous tubules appeared depleted of cellularity with an edematous interstitial space with few Leydig cells. Arsenic disrupts meiosis and post-meiotic stages of spermatogenesis (Gang et al., 2015). This severe damage, in this study, is consistent with the previous reports, (Li et al., 2012; Jahan et al., 2015; Zubair et al., 2017). These degenerative changes may be attributed to many factors which may be interrelated to each other, arsenic treatment produces degenerative changes in the germ cells and inhibits androgen production acting primarily at the level of pituitary to inhibit the release of LH and FSH (Jana et al., 2006), oxidative damage (Da Silva et al., 2016), reduction of testosterone levels from Leydig cells (Alamdar et al., 2017) and the down regulation of Ddx3y gene and protein expressions in testis and epididymis might be a mechanism for As-induced inhibition of spermatogenesis, sperm development, and sperm maturation (Li et al., 2012). Co-administration of Sp revealed a protection against these degenerative changes seen in the histopathology of As-treated rats and showed a normal spermatogenesis structure inside the seminiferous tubules.
in the present study, the histopathological alterations observed in arsenic intoxicated rats were consistent with the previous study (Maji et al., 2016), where presented liver with distorted hepatic structure, dilatation and congestion of central vein and hepatic sinusoids, activation of Kupffer cell, vacuolar degeneration of hepatocytes and focal areas of hepatic necrosis associated with mononuclear cells infiltration. Hepatic necrosis may be due to oxidative stress induced by arsenic that further involved in the cellular protein degradation. Shrinkage and necrosis of hepatocytes as a result of arsenic toxicity could increase the permeability and leakage of cellular material resulting in sinusoidal expansion. (Amer et al., 2016). These findings it is shown that they were prevented when Sp co-administered and the hepatic structure appeared in almost normal architecture. In accordance with previous studies on arsenic induced hepatotoxicity in goats co- treated with spirulina (Ghosh et al., 2014) significant hepatoprotective effects were observed.
This study revealed different histopathological alterations in the kidney of arsenic treated rats which described as vacuolar degeneration of epithelial lining renal tubules, congestion of renal blood vessels, focal necrosis of epithelial lining renal tubules associated with mononuclear cells infiltration as well as cystic dilatation of some renal tubules. These were in agreement with Ferzand et al. (2008), who explain the severe tubular necrosis which might be due to degradation of leaking cytoplasmic material and denaturing of protein components causing cytoplasmic vacuolation. These effects were reduced when spirulina was co-administered with arsenic treatment. In accordance with previous studies on arsenic induced nephrotoxicity in goats supplemented with spirulina (Ghosh et al., 2014), significant reno-protective effects were observed.
Results of the present study indicated the strong caspase-3 immunoreactivity in testes, liver and kidneys tissues of As-treated rats. It is evident from previous studies that apoptosis is involved in As-induced toxicity (Prakash and Kumar, 2016). Moreover, in this study the co-treatment with spirulina reduces the induced apoptosis by inhibiting caspase-3 activity, and this result was agree with Khafaga and El-Sayed (2018). This finding might be attributed to that the Spirulina had anti-mutagenic effect, which minimizes DNA damage and antiapoptitic effect (Chu et al., 2010).
The exact cause of this protective role of spirulina in recovering tissue damages is not fully understood. However, it is known that spirulina is an enriched source of nutrients like protein, amino acid, iron, β-carotene, phycocyanin, γ-lenolenic acid, vitamin B1, B2, B3, B6, B12 and essential fatty acid which are very much helpful to maintain the normal health. So, these findings indicate that spirulina has the positive role in decreasing the increased biochemical parameters due to arsenic toxicities also (Sayed et al., 2015).
Micronutrients and antioxidants components of spirulina have a significant role in the treatment of chronic arsenic poisoning. Ascorbic acid, Vitamin A , zinc, iron, spirulina, lipoic acid, ascorbic acid and α-tocopherol all have got ameliorating role against chronic arsenic poisoning (Korany et al., 2019). It has been observed in previous studies that alpha-lipoic acid, ascorbic acid and alphatocopherol have synergistic activity in decreasing tissue arsenic load. As spirulina is a rich source of vitamins, minerals and micronutrients, and vitamin C, all of which possess antioxidant properties. Thus, it can be suggested that a combination of vitamins, minerals, antioxidants and other micronutrients could be a novel therapeutic measure for the prevention of arsenicosis (Hasan et al., 2015).
Conclusion
Sodium arsenate induced toxicity in different organs of male rats could be successfully mitigated by co-adminstration of Spirulina platensis. Furthermore, the hormonal, biochemical and histological changes of the testicular, hepatic and renal tissues were ameliorated by co-treatment with Spirulina platensis.
Conflict of interest
The authors declare that they have no conflict of interests.
Authors’ contributions’
All authors contributed equally in the planning of the study, drafting the manuscript. All of them approve the final version of the article.
References