Advances in Animal and Veterinary Sciences
Research Article
Impacts of Housing and Storage Environments on Physical Quality and the Potential Public Health Risks of Chicken Table Eggs
Karima Mogahed Fahim1, Mahmoud Abdelaty Mahmoud Khalf2, Sara Mohamed Nader3, Elshaimaa Ismael2*
1Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt. 2Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt; 3Department of Zoonoses, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt.
Abstract | The freshness and safety of table eggs are important features that affect consumers’ choices. A total of 210 fresh and stored table eggs were sampled from nine-layer hen flocks raised in 6 cage houses and 3-deep-litter houses. Egg measurements of external and internal quality were performed on 60 eggs, while the microbiological examination was performed on 150 eggs (every 3 eggs were examined as a composite). Additional samples from floor litter, cage swabs, feces, and ovaries were collected for bacteriological isolation. Molecular detection of genes encoding virulence factors in Escherichia coli and Staphylococcus aureus isolates were performed by PCR. Results of egg quality measurements revealed significant reductions in specific gravity, albumin index, and Haugh unit of stored eggs when compared to fresh eggs (P < 0.05). Storing eggs either at > 25°C or within 20 -22°C led to the deterioration of egg quality parameters but didn’t affect microbial load when compared with fresh eggs (P > 0.05). Deep litter housing environment significantly raised the counts of aerobic bacteria (R2 = 0.70, P = 0.003), and Staphylococcus spp. (R2 = 0.91, P < 0.0001) on eggshells. Virulence genes stx1, eae, hlyA, and stx2 of E. coli were detected in feces, ovaries, and on eggshells of composites collected from cage houses. While Staphylococcus aureus MecA was detected on eggshells and internal content of egg composites collected from deep litter houses. Obtained results revealed the critical role of storage temperature on egg quality parameters, as well as, the great influence of the housing environment on the microbial profile of produced eggs. Regular monitoring and corrective control measures should be set to maintain egg quality and safety at acceptable levels.
Keywords | Deep litter; Cages; Virulence genes; Escherichia coli; Staphylococcus aureus
Received | March 12, 2021; Accepted | March 17, 2021; Published | July 01, 2021
*Correspondence | Elshaimaa Ismael, Department of Veterinary Hygiene and Management, Faculty of Veterinary Medicine, Cairo University, Giza 12211, Egypt; Email: elshaimaavet@cu.edu.eg
Citation | Fahim KM, Khalf MAM, Nader SM, Ismael E (2021). Impacts of housing and storage environments on physical quality and the potential public health risks of chicken table eggs. Adv. Anim. Vet. Sci. 9(8): 1176-1189.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1176.1189
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ismael et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Chicken eggs are the best source of cheap and high-quality protein (Farrell, 2013). For the first time in history, world egg production increased to more than 1338 billion eggs in 2015 (Conway, 2016). In intensive egg production systems laying hens are housed either in battery cages or on deep litter floor. In cage systems, eggs are physically separated from hens and manure, while in deep litter houses, eggs are in contact with litter and hens’ manure contributing to an increase in shell contamination and microbial contents (Hannah et al., 2011). Hence, the type of housing could affect the microbial quality and safety of table eggs.
Egg quality focuses on the external and internal egg parameters. The external quality of eggs includes the egg weight, volume, shell characteristics, and specific gravity, while internal quality involves the albumen and the yolk indices (Şekeroğlu and Duman, 2014; Ogunwole et al., 2015). Storage of table eggs for a prolonged time results in egg quality deterioration. Moreover, egg storage at high temperatures causes further loss to the eggs’ quality (Akter et al., 2014).
Although table eggs could get contaminated with different microorganisms, a few studies focused on bacterial contamination of table eggs. Table eggs could be infected before egg-laying due to diseased hens with infected ovaries. However, many studies estimated that the greatest source of microbial contamination of eggshells occurred shortly after laying due to contact with the contaminated environment (Senbeta et al., 2015; Merino et al., 2019). Increasing the level of microbial load on the eggshell consequently increases the chance of penetration of microorganisms into internal egg content (De Reu et al., 2006; Sodagari et al., 2019). The most isolated enteric and environmental egg contaminants were Staphylococcus spp., Salmonella spp., coliforms, yeast, and mold (De Reu et al., 2008; Chaemsanit et al., 2015). Most of these microorganisms are considered public health hazards and may cause food poisoning (Adesiyun et al., 2006; Adesiyun et al., 2007). Reducing the cases of egg-borne infections requires on-farm control strategies and proper handling and storage of table eggs.
The current study aimed to evaluate the effect of storage on physical egg quality and the microbial load, as well as, determine the impact of housing systems on the microbial quality and safety of table eggs.
MATERIALS AND METHODS
Layer hen flocks
Nine-layer hen flocks from Giza governorate, Egypt, were included in the study, during the period between April 2018 till August 2019. Out of the nine flocks, 6 of which were housed in a battery cage system, while the other three were housed on a deep-litter floor system. The raised hens were 29 to 60 weeks of age and at different stages of lay.
Sampling
Eggs: A total of 210 table eggs were collected using the simple random sampling method from the battery cage system (6 flocks, n= 135 eggs) and the deep litter system (3 flocks, n= 75 eggs). Both fresh (n= 105) and stored (n= 105) eggs were collected from each flock. Deep litter houses stored eggs at room temperature (> 25 °C), while cage houses stored eggs either at room temperature (> 25 °C) or at cooling temperature (20 – 22 °C). Egg quality measurements were performed on 60 eggs (30 fresh and 30 stored eggs), while the microbiological examination was performed on 150 eggs (every 3 eggs were examined as a composite).
Internal organs: Ten ovaries from 3 deep litter and 2 cage system farms were aseptically sampled during the post-mortem examination from 3 and 7 laying hens’ carcasses; respectively, then transferred to the laboratory in sterile cups.
Environmental samples: For evaluating the microbial load of the egg production environment, 12 battery cages were swabbed by cotton swabs, and 3 litter samples were collected from floor houses in sterile polyethylene bags. Fecal samples from one cage system flock were collected in sterile polyethylene bags.
All samples were aseptically collected and taken to the laboratory in an icebox for further analysis.
Egg quality measurements
Egg weight, volume, specific gravity, shape index, shell weight, and egg surface area were measured as external egg qualities (Alsaffar et al., 2013).
For testing internal egg qualities, eggs were broken and yolk weight and percentage, yolk index, albumen weight, and percentage, albumen index, and Haugh unit score were determined according to Hisasaga (2020).
Microbiological examination
Microbial count and isolation: The microbiological examination was conducted on 50 composite egg samples (Composite samples resembling three eggs each). Each composite egg sample was rinsed in 60 ml of sterile 0.1% peptone water (Lab M, 104) in sterile plastic bags and rinsed by shaking for 2 min (Al-Ajeeli, 2013). The egg rinse solution, litter samples, battery cage swabs, and fecal samples were tenth fold serially diluted with sterile peptone water up to 104 for eggs and 1010 for other environmental samples. Composite samples of the internal content of eggs (the content of three eggs/composite) and ovaries were collected in sterile flasks and thoroughly mixed for further microbial isolation.
All prepared samples and dilutions were subjected to microbiological examination according to (BAM 2013); for counting total aerobic bacteria (Standard Plate Count Agar, SPCA, OxoidTM CM0463), Coliforms (Eosin Methylene Blue agar plates, EMB, OxoidTM CM0069), Staphylococci (Baird-Parker medium supplemented with egg yolk tellurite emulsion 3.5%, Lab MTM LAB085), and total yeast and mold [Sabaroud Dextrose Agar, SDA, OxoidTM CM0463)]. All plates were incubated at 37°C for 24-48 h, except SDA plates were kept at 25°C for 3-5 days.
Furthermore, isolation and identification of E. coli, S. aureus, and Salmonella species were performed. Isolation of E. coli was carried out by streaking onto Levine’s Eosin Methylene Blue plates (L-EMB), followed by incubation
Table 1: Components and amplification programs of multiplex PCR used for detection of genes encoding virulence factors.
| Infectious agent |
Genes encoding virulence factors |
PCR components and volume (μl) |
Amplification programs | Reference |
|
E. coli
|
eae, hlyA, stx1 |
5μl Master Mix 0.5μl of each primers F&R (with total 3μl) 5μl DNA template 12μl PCR grade water |
1cycle 94°C for 5 min 35 cycles 94°C for 30 sec 62°C for 30 sec 72°C for 1 min 1cycle 72°C for 5 min |
Chandra et al., 2013 |
| stx2 |
5μl Master Mix 1μl of primer F&R (with total 2μl) 5μl DNA template 13μl PCR grade water |
1cycle 95°C for 3 min 35 cycles 95°C for 20 sec 58°C for 40 sec 72°C for 90 sec 1cycle 72°C for 5 min |
Gannon et al., 1992 |
|
|
Staphylococcus aureus |
mecA |
1X Master mix 20 pmol of each primer 5μl of DNA |
1 cycle 94◦c for 4min 35 cycles 94◦c for 1 min, 55◦c for 1 min, 72◦c for 1min 1 cycle 72◦c for 10 min. |
Asfour and Darwish 2011 |
Table 2: Primer sequences, their specific targets and amplicon sizes
| Primer name | Primer sequence 5’-3’ (reference) | Product size | References |
| EAE (eae) |
F:TCAATGCAGTTCCGTTATCAGTT R:GTAAAGTCCGTTACCCCAACCTG |
482 |
Vidal et al. (2005) |
| Stx1 (stx1) |
F:CGATGTTACGGTTTGTTACTGTGACAGC R:AATGCCACGCTTCCCAGAATTG |
244 |
Müller et al. (2007) |
| HlyA(hlyA) |
F:AGCTGCAAGTGCGGGTCTG R:TACGGGTTATGCCTGCAAGTTCAC |
569 |
Wang et al. (2002) |
| Stx2 (stx2) |
F:GTTTTGACCATCTTCGTCTGATTATTGAG R:AGCGTAAGGCTTCTGCTGTGAC |
324 |
Müller et al. (2007) |
| mecA |
F:GTGAAGATATACCAAGTGATT R:ATGCGCTATAGATTGAAAGGAT |
147bp |
Asfour and Darwish (2011) |
Table 3: Comparison between fresh and stored eggs regarding their external and internal quality parameters (Mean±SE)
| Age of Eggs | |||||
| Fresh eggs | Stored eggs | df | t |
P - value |
|
| External characters: | |||||
| Egg weight (g) | 65.44±1.52 | 65.35±1.11 | 59 | 0.05 | 0.960 |
|
Egg Volume (cm3) |
60.07±1.52 | 61.97±1.38 | 59 | -0.93 | 0.358 |
| Specific gravity |
1.093±0.014 a |
1.059±0.009 b |
59 | 2.05 | 0.045 |
| Egg width (cm) | 4.50±0.04 | 4.45±0.03 | 59 | 1.10 | 0.275 |
| Egg length (cm) | 5.91±0.06 | 5.96±0.05 | 59 | -0.58 | 0.562 |
| Shape index (%) | 76.30±0.64 | 74.85±0.60 | 59 | 1.65 | 0.105 |
|
Egg surface area (cm2) |
85.28±1.46 | 85.17±1.09 | 59 | 0.06 | 0.954 |
| Shell weight (g) | 6.85±0.12 | 7.06±0.10 | 59 | -1.31 | 0.288 |
| Shell ratio (%) | 10.52±0.14 | 10.85±0.15 | 59 | -1.56 |
0.680 |
| Internal characters: | |||||
| Albumin weight (g) | 40.98±0.97 | 40.52±0.75 | 58 | 0.38 | 0.707 |
| Albumin ratio (%) | 62.68±0.54 | 62.16±0.80 | 58 | 0.54 | 0.595 |
| Albumin height (cm) |
0.82±0.03 a |
0.72±0.04 b |
58 | 2.04 | 0.045 |
| Albumin length (cm) |
9.55±0.21 b |
10.40±0.29 a |
58 | -2.35 | 0.022 |
| Albumin width (cm) | 7.88±0.15 | 8.46±0.27 | 44.83 | -1.90 | 0.064 |
| Albumin Ave. width (cm) | 8.71±0.17 | 9.12±0.40 | 40.13 | -0.95 | 0.346 |
| Albumin index (%) |
9.59±0.41 a |
7.83±0.72 b |
47.53 | 2.14 | 0.038 |
| Haugh unit |
88.68±1.56 a |
78.40±3.67 b |
40.43 | 2.58 | 0.014 |
| Yolk weight (g) | 17.62±0.62 | 17.72±0.74 | 58 | -0.11 | 0.912 |
| Yolk ratio (%) | 26.80±0.57 | 26.08±1.19 | 59 | 0.54 | 0.592 |
| Yolk height (cm) | 1.59±0.05 | 1.46±0.06 | 58 | 1.70 | 0.095 |
| Yolk width (cm) | 4.46±0.08 | 4.62±0.13 | 47.64 | -1.11 | 0.274 |
| Yolk Index (%) | 36.23±1.37 | 32.99±1.78 | 58 | 1.45 | 0.153 |
| Yolk Albumen ratio (%) | 43.09±1.33 | 42.69±2.43 | 46.41 | 0.14 |
0.888 |
a, b Different superscripts indicate significant difference at P < 0.05; SE: Standard error.
at 37°C for 24-48 h. Flat colonies with dark center and green metallic luster were streaked on agar slants and kept at 35°C incubation for 18 h (Fahim et al., 2019). Biochemical identification was done as described by Da Silva et al. (2018). For isolation of Salmonella spp., 25 ml of the original prepared sample was aseptically transferred to 225 ml of sterile buffered peptone water and incubated at 37°C for 16-20 h. A loopful from each of the previous pre-enriched broth tubes were inoculated into a sterile tube containing 10 ml Rappaport Vassiliadis broth, then incubated at 43°C for 24 h. A loopful from each Rappaport Vassiliadis enriched tube was streaked on a dried surface of Xylose Lysin Deoxycholate agar (XLD). Inoculated plates were incubated at 37°C for 48 hours (Andrews et al., 2018). For isolation and identification of S. aureus, 5 typical and atypical colonies were selected from each plate, then identified according to (BAM, 2013).
Molecular detection of genes encoding virulence factors in E. coli and S. aureus isolates
DNA was extracted from the bacterial colonies by the boiling method. Bacterial strains were grown in brain heart infusion broth at 37°C overnight. Organisms from 1.5 ml growth were pelleted by centrifugation at 1200xg for 10 min. The bacterial pellet was resuspended in 150μl of sterile distilled water. Lysis of bacteria was done by boiling in a water bath for 10 min. The lysate was centrifuged again as before, and the supernatant was used as a template for polymerase chain reaction as stated in Tables (1 and 2) (Wani et al., 2003). The PCR product was run on 1.5% agarose gel at 80 volts for 1 hour. The DNA bands were visualized using an Ultraviolet lightbox with a camera (Gel Doc 2000, BIO-RAD) and photographed. The expected size of the PCR products for virulence encoding gene of E. coli and mecA genes was estimated concerning 50 bp DNA ladder (Jena Bioscience).
Statistical analysis
Data analysis was performed using PASW Statistics Version 18.0 software (SPSS Inc., Chicago, IL, USA). Independent sample t-test was conducted to compare the effect of storage and housing type on physical and microbial qualities of eggs and expressed as means ± SE. One-way analysis of variance (ANOVA) was tested to compare the effect of season on physical quality of eggs and expressed as means ± SE. The statistical model formula was:
Yij = μ + αi + ϵij
where Yij indicated the measurement of egg quality parameters. μ was the grand mean, αi represented the season’s effect, and ϵij stated the random error.
Kruskal-Wallis H and Mann–Whitney U tests were used when data were not normally distributed. Pearson correlation (r) and linear regression (R2) were used to test the association between environmental and eggshell contamination. Chi-square test for independence (χ2) and Fisher’s Exact test (FET) was performed to test the relation between different samples and the rate of microbial isolation. Significance was set at P-value < 0.05.
RESULTS
Egg physical quality
Results in Tables (3 and 4) showed that the specific gravity of fresh eggs was greater than that of stored eggs. This difference was significant (t(59) = 2.05, P = 0.045). The specific gravity of eggs stored at room temperature (> 25°C) was greater than that of eggs stored at a cooler temperature (20-22°C). This difference was significant (t(29) = 2.37, P = 0.025). Similarly, the albumin height of fresh eggs was
Table 4: Effect of different storage temperatures (°C) on the external and internal egg quality (Mean±SE).
| Storage condition (°C) | |||||
| Room temp. (> 25 °C) | Cooling temp.(20-22 °C) | df | t |
P - value |
|
| External characters: | |||||
| Egg weight (g) | 64.41±1.37 | 68.05±1.45 | 29 | -1.46 | 0.155 |
|
Egg Volume (cm3) |
60.30±1.51 b |
66.75±2.62 a |
29 | -2.16 | 0.039 |
| Specific gravity |
1.071±0.009 a |
1.025±0.022 b |
29 | 2.37 | 0.025 |
| Egg width (cm) | 4.44±0.03 | 4.49±0.05 | 29 | -0.79 | 0.438 |
| Egg length (cm) |
5.91±0.06 b |
6.10±0.07 a |
20.48 | -2.11 | 0.047 |
| Shape index (%) | 75.29±0.74 | 73.60±0.88 | 29 | 1.24 | 0.226 |
|
Egg surface area (cm2) |
84.17±1.32 | 88.05±1.60 | 29 | -1.59 | 0.123 |
| Shell weight (g) | 6.98±0.11 | 7.28±0.25 | 29 | -1.30 | 0.203 |
| Shell ratio (%) | 10.89±0.18 | 10.71±0.32 | 29 | 0.51 | 0.613 |
| Internal characters: | |||||
| Albumin weight (g) | 40.77±0.84 | 39.70±1.72 | 28 | 0.60 | 0.556 |
| Albumin ratio (%) | 63.37±0.55 | 58.20±2.48 | 6.61 | 2.03 | 0.084 |
|
Albumin height (cm) |
0.73±0.03 | 0.69±0.15 | 6.64 | 0.29 | 0.783 |
| Albumin length (cm) | 10.25±0.26 | 10.89±0.92 | 7.02 | -0.67 | 0.526 |
| Albumin width (cm) | 8.08±0.21 | 9.71±0.79 | 6.86 | -1.99 | 0.087 |
| Albumin Ave. width (cm) | 9.16±0.22 | 10.30±0.84 | 6.81 | -1.31 | 0.234 |
| Albumin index (%) | 8.20±0.56 | 7.73±2.46 | 6.64 | 0.19 | 0.858 |
| Haugh unit | 83.22±2.05 | 73.76±9.21 | 6.60 | 1.00 | 0.351 |
| Yolk weight (g) |
16.66±0.66 b |
21.20±1.85 a |
28 | -2.92 | 0.007 |
| Yolk ratio (%) | 25.74±0.63 | 30.94±2.48 | 6.80 | -2.03 | 0.083 |
| Yolk height (cm) |
1.57±0.03 a |
1.11±0.18 b |
6.23 | 2.50 | 0.045 |
| Yolk width (cm) | 4.41±0.08 | 5.31±0.39 | 6.45 | -2.26 | 0.062 |
| Yolk Index (%) | 35.86±0.79 | 23.54±6.19 | 6.20 | 1.97 | 0.094 |
| Yolk Albumen ratio (%) | 40.88±1.39 | 54.75±6.20 | 6.62 | -2.18 |
0.68 |
a, b Different superscripts indicate significant difference at P < 0.05; SE: Standard error.
Table 5: Effect of season on the external and internal egg quality (Mean±SE).
| Season | |||||
| Winter | Spring | Summer |
F2,58 |
P-value |
|
| External characters: | |||||
| Egg weight (g) | 64.94±2.08 | 64.60±1.25 | 68.46±1.07 | 1.36 | 0.265 |
|
Egg Volume (cm3) |
60.57±2.57ab |
59.60±1.27b |
66.33±1.69a |
3.61 | 0.033 |
| Specific gravity | 1.08±0.02 | 1.09±0.01 | 1.04±0.02 | 2.97 | 0.059 |
|
Egg width (cm) |
4.49±0.07 | 4.48±0.03 | 4.48±0.03 | 0.01 | 0.989 |
| Egg length (cm) |
5.86±0.11ab |
5.89±0.05b |
6.14±0.04a |
3.53 | 0.036 |
| Shape index (%) |
76.67±1.37a |
76.14±0.53a |
72.90±0.66b |
5.05 | 0.009 |
|
Egg surface area (cm2) |
84.09±2.52 | 84.48±1.19 | 88.50±0.83 | 1.67 | 0.198 |
|
Shell weight (g) |
7.04±0.16 | 6.90±0.10 | 7.10±0.19 | 0.56 | 0.573 |
| Shell ratio (%) | 10.87±0.19 | 10.74±0.14 | 10.37±0.21 | 1.12 | 0.333 |
| Internal characters: | |||||
| Albumin weight (g) | 42.61±1.50 | 40.87±0.78 | 39.23±0.89 | 1.21 | 0.304 |
| Albumin ratio (%) |
65.60±0.70a |
63.32±0.37a |
57.39±1.05b |
29.13 | <0.0001 |
| Albumin height (cm) |
1.13±0.05a |
0.76±0.02b |
0.61±0.05c |
32.24 | <0.0001 |
| Albumin length (cm) |
8.44±0.26c |
9.84±0.18b |
11.36±0.41a |
13.93 | <0.0001 |
| Albumin width (cm) |
7.30±0.17b |
7.92±0.14b |
9.56±0.39a |
16.23 | <0.0001 |
|
Albumin Ave. width (cm) |
7.87±0.20c |
8.88±0.15b |
10.46±0.36a |
17.46 | <0.0001 |
| Albumin index (%) |
14.45±0.95a |
8.70±0.33b |
6.02±0.60c |
33.10 | <0.0001 |
| Haugh unit |
103.54±2.11a |
85.17±1.23b |
72.48±4.00c |
25.46 | <0.0001 |
| Yolk weight (g) |
15.29±0.72b |
16.83±0.49b |
22.00±0.77a |
16.50 | <0.0001 |
| Yolk ratio (%) |
23.54±0.74b |
25.94±0.41b |
32.13±0.99a |
29.03 | <0.0001 |
|
Yolk height (cm) |
1.79±0.04a |
1.62±0.02a |
1.04±0.08b |
63.82 | <0.0001 |
| Yolk width (cm) |
4.06±0.09b |
4.40±0.06b |
5.30±0.17a |
28.46 | <0.0001 |
| Yolk Index (%) |
44.19±1.60a |
37.03±0.53b |
20.40±2.16c |
79.33 | <0.0001 |
| Yolk Albumen ratio (%) |
35.98±1.52b |
41.17±0.91b |
56.60±2.74a |
30.97 |
<0.0001 |
a, b, c Different superscripts indicate significant difference at P < 0.05; SE: Standard error.
Table 6: Effect of housing and storage on the microbial count of eggshells (log10 CFU/ml)
|
Number of egg composites (N=50) |
Incidence No. (%) |
Range (log10 CFU/ml) |
Mean log10 CFU/ml ± SE |
||
| Min. | Max. | ||||
| Total Aerobic Bacteria | |||||
| Deep litter | Fresh (n= 9) | 9 (100%) | 3.54 | 6.10 |
4.39± 0.28 a |
| Stored (n= 9) | 9 (100%) | 3.15 | 6.18 |
4.74± 0.36 a |
|
| Cages | Fresh (n= 16) | 16 (100%) | < 1.00 | 4.28 |
2.42± 0.22 b |
| Stored (n= 16) | 16 (100%) | < 1.00 | 3.34 |
2.32± 0.24 b |
|
|
P < 0.0001 |
|||||
| Total Staphylococci | |||||
| Deep litter | Fresh | 9 (100%) | 2.90 | 6.02 |
4.16± 0.31 a |
| Stored | 9 (100%) | 3.00 | 6.11 |
4.53± 0.37 a |
|
| Cages | Fresh | 11 (68.8%) | < 1.00 | 3.93 |
1.86± 0.34 b |
| Stored | 14 (87.5%) | < 1.00 | 3.84 |
2.59± 0.27 b |
|
|
P < 0.0001 |
|||||
| Total Coliform count | |||||
| Deep litter | Fresh | 6 (66.7%) | < 1.00 | 4.60 |
1.93±0.56 ab |
| Stored | 6 (66.7%) | < 1.00 | 4.43 |
2.50±0.64 a |
|
| Cages | Fresh | 3 (18.6%) | < 1.00 | 4.34 |
0.73±0.39 b |
| Stored | 4 (25.0%) | < 1.00 | 3.92 |
0.78±0.32 b |
|
|
P = 0.033 |
|||||
| Total fungal count | |||||
| Deep litter | Fresh | 9 (100%) | < 1.00 | 4.48 |
2.80± 0.39 ab |
| Stored | 9 (100%) | 2.00 | 4.13 |
3.15± 0.23 a |
|
| Cages | Fresh | 9 (56.2%) | < 1.00 | 4.60 |
1.59± 0.41 bc |
| Stored | 7 (43.8%) | < 1.00 | 4.92 |
1.22± 0.39 c |
|
|
P = 0.004 |
|||||
a,b,c Different superscripts indicate significant difference at P < 0.05; SE: Standard error.
greater than that of stored eggs. This difference was significant (t(58) = 2.04, P = 0.045). The albumin length of stored eggs was greater than that of fresh eggs. This difference was significant (t(58) = 2.35, P = 0.022). The albumin index (%) of fresh eggs was greater than that of stored eggs. This difference was significant (t(47.53) = 2.14, P = 0.038). Haugh unit of fresh eggs was greater than that of stored eggs, this difference was significant (t(40.43) = 2.58,
Table 7: Effect of storage temperature on the microbial load of stored shell eggs from cage system (log10 CFU/ml)
|
Number of egg composites (N=16) |
Incidence No. (%) |
Range (log10 CFU/ml) |
Mean log10 CFU/ml ± SE |
|
| Min. | Max. | |||
| Total Aerobic Bacteria | ||||
| Room temp. (> 25 °C) (n=8) | 7 (87.5%) | < 1.00 | 3.34 |
2.47±0.37 a |
|
Cooling temp. (20-22 °C) (n=8) |
7 (87.5%) | < 1.00 | 3.00 |
2.17±0.33 a |
|
P = 0.184 |
||||
| Total Staphylococci | ||||
| Room temp. (> 25 °C) | 6 (75.0%) | < 1.00 | 3.23 |
2.10±0.47 a |
| Cooling temp. (20-22 °C) | 8 (100%) | 2.70 | 3.84 |
3.08±0.14 a |
|
P = 0.051 |
||||
| Total Coliform count | ||||
| Room temp. (> 25 °C) | 1 (12.5%) | < 1.00 | 2.60 |
0.83±0.41 a |
| Cooling temp. (20-22 °C) | 1 (12.5%) | < 1.00 | 3.92 |
0.74±0.52 a |
|
P = 0.700 |
||||
| Total fungal count | ||||
| Room temp. (> 25 °C) | 9 (50.0%) | < 1.00 | 2.78 |
1.53±0.46 a |
| Cooling temp. (20-22 °C) | 2 (25.0%) | < 1.00 | 4.92 |
0.90±0.64 a |
|
P = 0.224 |
||||
a,b Different superscripts indicate significant difference at P < 0.05 using Mann-Whitney Test; SE: Standard error.
Table 8: Effect of season on the microbial load of eggs from cage system (log10 CFU/ml)
|
Number of egg composites (N=32) |
Incidence No. (%) |
Range (log10 CFU/ml) |
Mean log10 CFU/ml ± SE |
|
| Min. | Max. | |||
| Total Aerobic Bacteria | ||||
| Winter (n=8) | 8 (100%) | 2.30 | 4.28 |
2.86±0.22 a |
| Spring (n=12) | 11 (91.7%) | < 1.00 | 3.34 |
2.34±0.24a |
| Summer (n=12) | 10 (83.3%) | < 1.00 | 3.32 |
2.06±0.30a |
|
P = 0.154 |
||||
| Total Staphylococci | ||||
| Winter (n=8) | 7 (87.5%) | < 1.00 | 3.93 |
2.67±0.42a |
| Spring (n=12) | 8 (66.7%) | < 1.00 | 2.78 |
1.69±0.36b |
| Summer (n=12) | 10 (83.3%) | < 1.00 | 3.84 |
2.47±0.36a |
|
P = 0.025 |
||||
| Total Coliform count | ||||
| Winter (n=8) | 6 (75.0%) | < 1.00 | 4.34 |
2.52±0.61a |
| Spring (n=12) | 1 (8.3%) | < 1.00 | 2.00 |
0.17±0.17b |
| Summer (n=12) | 1 (8.3%) | < 1.00 | 2.00 |
0.17±0.17b |
|
P < 0.0001 |
||||
| Total fungal count | ||||
| Winter (n=8) | 5 (62.5%) | < 1.00 | 4.92 |
2.38±0.81a |
| Spring (n=12) | 11 (91.7%) | < 1.00 | 3.30 |
2.21±0.23a |
| Summer (n=12) | 0 | < 1.00 | < 1.00 |
0.00b |
|
P < 0.0001 |
||||
a,b Different superscripts indicate significant difference at P < 0.05 using Kruskal Wallis Test; SE: Standard error.
Table 9: Microbial load of housing environment (Mean log10 CFU/ml)
|
Mean log10 CFU/ml ±SE |
|||||
| Deep litter sample |
Cage swabs |
df | t |
P - value |
|
| Total Aerobic Bacteria |
9.34±0.19 a |
2.26±0.14 b |
4 | 30.55 | < 0.0001 |
| Total Staphylococci |
8.13±0.75 a |
1.33±0.67 b |
4 | 6.75 | 0.003 |
| Total Coliform |
6.98±0.06 a |
1.99±0.99 b |
4 | 5.04 | 0.037 |
| Salmonella species | 1/3 (33.3%) | 6/6 (100%) | FET | 0.083 | |
a,b Different superscripts within the same row indicate significant difference at P < 0.05; SE: Standard error; FET: Fisher’s Exact Test.
P = 0.014).
Results of Table (5) showed that the egg volume and shape index differed significantly concerning the season of the year (P = 0.033 and 0.009, respectively). Surprisingly, all internal parameters of examined eggs (except albumin weight) displayed significantly better qualities for eggs produced during the winter season compared to those laid during the summer season (P < 0.0001). However, yolk indices were significantly greater for eggs laid in winter than those of summer season (P < 0.0001).
Egg microbial quality
Results of the effect of housing on total aerobic bacteria, Staphylococcus, coliforms, and fungal counts of eggshells produced in deep litter and battery cage housing systems were displayed in Table (6). Results showed a significant effect of housing type on the total aerobic bacterial count of eggshells, (P < 0.0001). Similarly, a significant effect of housing type on the total Staphylococcus count of eggshells was found (P < 0.0001). Likewise, a significant effect of housing type on the total eggshells’ coliforms count was found (P = 0.033); Kruskal-Wallis H test. Additionally, there was a significant effect of housing type on eggshells’ total fungal count (P = 0.004). Post hoc comparisons using the Tukey HSD test indicated that the mean microbial counts of eggshells produced from deep litter housing systems were significantly higher than eggshells produced from battery cage housing systems.
A Kruskal-Wallis H test showed that stored eggs produced from the deep-litter system showed a significantly higher coliform count on eggshell than that of fresh eggs and eggs produced from cage systems (P = 0.033).
Results in Table (7) demonstrated the effect of storage temperature on the eggshell microbial count of the stored eggs from the cage system. Mostly, no significant effect on total aerobic bacteria count (P = 0.184), Staphylococcus count (P = 0.051), coliforms count (P = 0.700), or fungal count (P = 0.224) was observed when eggs stored in room temperature (> 25°C) or in 20-22 °C.
Table (8) displayed the relationship between the season and the microbial loads of eggshells. All eggshell contaminants reported the highest levels during the winter season. However, the significant differences were observed in Staphylococcus count (P = 0.025), coliforms count (P < 0.0001), and fungal count (P < 0.0001), while total aerobic bacteria count didn’t differ significantly due to season (P = 0.154).
From the data in Table (9), an independent sample t-test showed that the total aerobic bacterial count of litter samples was greater than counts of cages’ swabs. This difference was significant (P < 0.0001). As well, the Staphylococcus count of litter samples was greater than that of cages’ swabs. This difference was significant (P =0.003). Moreover, the coliforms count of litter samples was greater than that of cages’ swabs. This difference was significant (P =0.037). Salmonella spp. was isolated from 33.3% of litter samples and 100% of cage swabs (P =0.083).
Results of Pearson correlation indicated that there was a strong positive association between environmental and eggshell aerobic bacterial counts (r = 0.84, P = 0.001). For each 1 log10 increase in environmental bacterial count, we observed a 0.23 log10 CFU increase in eggshell aerobic bacterial count (P =0.003, R2 = 0.70); as demonstrated in Figure (1-A). Similarly, there was a strong positive association between environmental and eggshell Staphylococcus counts (r = 0.95, P < 0.0001). For each 1 log10 increase in environmental Staphylococcus count, we observed a 0.51 log10 CFU increase in eggshell Staphylococcus count (F(1, 8) = 78.09, P < 0.0001, R2 = 0.91); as demonstrated in Figure (1-B). However, there was weak association between environmental and eggshell coliform counts (r = 0.48, P = 0.081). For each 1 log10 increase in environmental coliform count, we observed a 0.15 log10 CFU increase in eggshell coliform count (P = 0.162, R2 = 0.23); as demonstrated in
Table 10: Incidence of microbial isolation from egg composites
| Samples | No. of samples |
No. (%) of positive samples |
||||
| Salmonella | Staphylococci spp. | Coliforms | Fungi | P-value | ||
| Ovaries | 10 |
8 (80.0) a |
2 (20.0) b |
2 (20.0) b |
2 (20.0) b |
0.012 |
| Deep litter | 3 | 1/3 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cages | 7 | 7/7 (100) | 2 (28.6) | 2 (28.6) | 2 (28.6) | |
| Fecal matter (cages) | 1 | 1 (100) | 1 (100) | 1 (100) | 1 (100) | |
| Egg composites: | ||||||
| Shell | ||||||
| Fresh egg | 25 |
5 (20.0) b |
20 (80.0) a |
9 (36.0) b |
18 (72.0) a |
< 0.0001 |
| Stored egg | 25 |
9 (36.0) b |
23 (92.0) a |
10 (40.0) b |
16 (64.0) b |
< 0.0001 |
| Total | 50 |
14 (28.0) c |
43 (86.0) a |
19 (38.0) c |
34 (68.0) b |
< 0.0001 |
| Internal content | ||||||
| Fresh egg | 25 | 2 (8.0) | 3 (12.0) | 1 (4.0) | 2 (8.0) | 0.866 |
| Stored egg | 25 | 1 (4.0) | 4 (16.0) | 0.0 | 0.0 | 0.349 |
| Total | 50 |
3 (6.0) |
7 (14.0) |
1 (2.0) |
2 (4.0) |
0.144 FET |
| Eggs/House type | ||||||
| Deep litter | 18 | 7 (38.8) |
18 (100) A |
12 (66.7) A |
18 (100) A |
|
| Cages | 32 | 7 (21.9) |
25 (78.1) B |
7 (21.9) B |
16 (50) B |
|
|
P-value |
0.325 | 0.040 | 0.002 |
< 0.0001 |
||
a, b Different superscripts in the same row indicate significant difference at P < 0.05; A, B Different superscripts in the same column indicate significant difference at P < 0.05; FET: Fisher’s Exact Test.
Table 11: Prevalence of E. coli and S. aureus virulence genes in layer farms
| E. coli | |||||||
| Samples |
E. coli (%) |
Virulent E. coli (%) |
Virulence genes | ||||
| Eggs | stx1 | eae | hlyA | stx2 | |||
| Eggshells (cages): | 9/32 (28.1) | 6/32 (18.8) | |||||
| Fresh | 5/16 (31.3) | 3/16 (18.8) |
1st |
+ | + | ||
|
2nd |
+ | + | |||||
|
3rd |
+ | ||||||
| Stored | 4/16 (25.0) | 3/16 (18.8) |
1st |
+ | |||
|
2nd |
+ | ||||||
|
3rd |
+ | + | |||||
| Ovaries | 1*/10 (10.0) | 1*/10 (10.0) | + | ||||
| Fecal sample (cages) | 1/1 (100) | 1/1 (100) | + | + | + | + | |
| Cage swab | 1/6 (16.7) | 0/6 (0.0) | |||||
| S. aureus | |||||||
|
Staphylococcus aureus (%) |
Virulent Staph. aureus (%) |
Virulence genes (mec A) |
|||||
| Eggshells (deep litter): | 4/18 (22.2) | 4/18 (22.2) | |||||
| Fresh | 1/9 (11.1) | 1/9 (11.1) | + | ||||
| Stored | 3/9 (33.3) | 3/9 (33.3) | + | ||||
| Egg content (deep litter) | 1/9 (11.1) | 1/9 (11.1) | + | ||||
| Eggshells (cages): | 5/32 (15.6) | 0/32 (0.0) | |||||
| Fresh | 4/16 (25.0) | 0/16 (0.0) | |||||
| Stored | 1/16 (6.3) | 0/16 (0.0) | |||||
| Fecal sample (cages) | 0/1 (0.0) |
0/1 (0.0) |
|||||
* The positive E. coli ovarian sample were from hens housed in cage system.
Figure (1-C). Eventually, there was no association between environmental and eggshell fungal counts (r = - 0.06, P = 0.436), (P = 0.872, R2 = 0.003); as demonstrated in Figure (1-D).
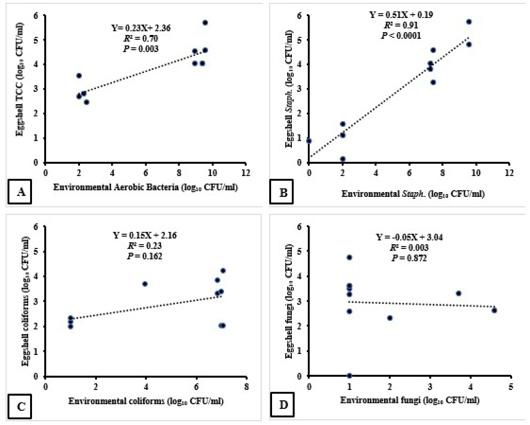
Figure 1: The association between the microbial load of the farm surface environment and the eggshell microbial contamination. R2: Coefficient of Determination.
Egg microbial isolates
Results in Table (10) displayed that Staphylococcus spp. was the most predominant eggshell contaminant isolated from both fresh (P < 0.0001) and stored (P < 0.0001) egg composites. The rate of isolation of Salmonella was relatively low and was found in the internal content of 3/50 (6.0%) egg composites and on the shell of (28.0%) egg composites. However, a high rate of Salmonella infections (80%) was observed in ovarian samples collected from both floor and cage housing systems. Additionally, eggs produced in deep litter houses showed significantly higher isolation rates of Staphylococcus spp. (P = 0.040), Coliforms (P = 0.002), and total fungi (P < 0.0001) than eggs produced from cage battery houses.
Molecular detection of genes encoding virulence factors from the bacterial isolates
Results listed in Table (11) reported the detection of E. coli virulence genes (eae, stx1, hlyA, and stx2) in 8 samples out of 12 positive E. coli isolates. A fecal sample from caged hens showed positivity to eae, stx1, hlyA, and stx2 virulence genes. Similarly, an ovary from a caged hen showed positivity to the hlyA virulence gene. Besides, eggs produced from cage houses revealed the presence of the stx2 viru lence gene on the eggshells of one fresh egg and two stored eggs composites. Combined stx1+hlyA, eae+hlyA, and stx1+stx2 virulence genes were detected in two fresh and one stored egg composites produced from cage system houses.
Furthermore, the Staphylococcus aureus virulence gene, mecA, was detected in 5 strains isolated from deep litter houses out of 11 Staphylococcus aureus strains. Interestingly, one fresh egg composite displayed Staphylococcus aureus virulence gene, mecA, from the external eggshell and the internal egg content. As well, the Staphylococcus aureus virulence gene, mecA, was detected on the eggshell of three stored egg composites produced from deep litter houses (Table 11).
DISCUSSION
Our results revealed the impact of storage on some quality parameters of table eggs. Table eggs are stored in layer farms to be marketed. According to FAO (2003), eggs should be stored in a clean and well-ventilated environment, with constant temperature and relative humidity. Moreover, regular monitoring of the interior quality of stored eggs should be done. Also, refrigerated storage is recommended for better egg quality.
Results showed that most of the internal quality parameters of fresh eggs, especially specific gravity, albumin index, and Haugh unit, were better than stored eggs. These findings were consistent with Stadelman and Cotterill (2007) and Eke et al. (2013), who stated that the escape of moisture and gases from within the eggs via egg pores results in egg weight loss. The carbonic acid of egg white breaks down into water and carbon dioxide gas, which escapes through pores, and egg white becomes watery. This loss of carbon dioxide during egg storage changes pH to the alkaline side, which loosens the mucin fibers of albumin, causing it to lose its strength and consequently decreasing the Haugh unit of the stored egg. Moreover, the water of watery albumin is absorbed by the egg yolk to equalize the pressure inside the egg, so the yolk becomes swollen and flabby in shape, and a lower yolk index is obtained.
Results of the physical qualities of stored eggs were almost similar either in eggs stored in ambient room temperature or slightly cooler conditions (20-22°C). Grashorn (2016) reported that at 15 - 22°C, there was a rapid deterioration of egg quality, including the Haugh unit, and recommended eggs to be stored at 6°C. The same findings were reported by Ibrahim et al. (2019); the long storage at room temperature or refrigerator deteriorated egg weights, internal qualities, and Haugh unit, although storing at room temperature revealed the lowest results. The reduction of the Haugh unit indicates the decline of lysozyme activity, which plays a vital role in protecting the egg contents from microbial contamination (Trziszka, 1994).
Regarding the effect of macroclimatic conditions on table egg qualities, we observed that eggs delivered in winter had higher freshness parameters than those laid in summer. Our findings were consistent with Gumułka et al. (2017), who reported greater egg weights, albumin%, albumin indices, Haugh units, and shell quality for eggs delivered in winter than those laid in summer. Furthermore, he stated that yolk quality was higher in summer than winter. It may be attributed to the stress of the higher temperatures in the summer season, which influence the metabolism and egg formation (Gumułka et al., 2017). Moreover, the season of the year could be a factor influencing the microbial load of table eggs as stated by Chousalkar et al. (2021), especially the degree of humidity that affects the fungal count over the eggshell.
From the microbiological point of view, stored eggs tested in this study didn’t show a significant difference from fresh eggs based on microbial load. However, the microbial quality of eggs differed significantly due to the type of production housing system. Eggs produced from deep litter housing showed a higher microbial load than eggs produced from battery cage houses. De Reu et al. (2008) stated in their study that the housing system has an effect on the microbial load of eggshells and that eggs produced from non-cage systems were higher in aerobic bacterial contaminants than eggs produced from various cage systems. Additionally, Hannah et al. (2011) discussed that houses separating laying hens from manure and shaving resulted in eggs with lower bacterial counts. These findings were relevant to the results of the microbial examination of surfaces of different housing systems tested in this study. In deep litter houses, laid eggs are in contact with hens and litter, which is a mixture of wood shaving, manure, and feathers. While in battery cage houses, eggs roll on the sloped cage wire into the collection trough away from contact with hens or manure. Results of microbial examination revealed that each 1g of deep litter had more than 6 log10 CFUs higher than cage wire swabs for aerobic bacteria and Staphylococcus counts. Similarly, Soliman et al. (2020) reported a significant difference (P = 0.001) in mean total bacterial counts of wood shaving litter (5.25±0.00 log10 CFU/ml) and battery swabs (4.14±0.01 log10 CFU/ml), as well as a significant difference (P < 0.0001) in mean total Enterobacteriaceae counts of wood shaving (3.74±0.02 log10 CFU/ml) and battery swabs (1.80±0.01 log10 CFU/ml). These findings agreed with Parisi et al. (2015), who reported that the longer the contact of eggs with hens and contaminated litter, the higher the bacterial load on eggshells compared to eggs produced from cage systems.
Results of the current study revealed that the predominant contaminant of eggshells and internal egg content was Staphylococcus spp. (86% and 14%, respectively), followed by total fungi (68% and 4%, respectively), then coliforms (38% and 2%, respectively). These frequencies agreed with EL-Kholy et al. (2020) who found Staphylococcus spp. in the internal content of 13% of examined eggs. However, Salmonella spp. was isolated in a relatively low frequency (28% and 6%, respectively), which agreed with what was reported by Musgrove et al. (2008). Though egg contamination with Salmonella is a critical public health risk, limited research studies focused on other bacterial contaminants that threaten public health, as noted by De Reu et al. (2008).
Bacterial contamination of eggs can occur via vertical or horizontal routes (European Food Safety Authority, EFSA, 2005). Hens have a common cloacal opening for intestinal, urinary, and reproductive tracts, which could contribute to eggshell contamination while the egg moves along this route. Furthermore, minor defects in the eggshell may provide access to bacteria into the internal egg contents (De Reu et al., 2006). Eggs could act as a vehicle for pathogens in the food chain, including extraintestinal pathogenic Escherichia coli (ExPEC) (Mitchell et al., 2015). However, the potential of eggs as sources of Diarrheagenic E. coli (DEC) has been investigated to a much less extent (Chousalkar et al., 2010).
Escherichia coli (E. coli) is a normal gut flora of birds and humans, and some E. coli strains have acquired virulence genes that enable them to induce diarrhea and other associated illnesses (Wani et al., 2004). The most important group of the zoonotic E. coli are the Diarrhoeagenic class that includes Enteropathogenic E. coli (EPEC), Enteroinvasive E. coli (EIEC), Enteroaggregative E. coli (EAEC), Enterotoxigenic E. coli (ETEC), and Shiga toxin-producing E. coli (STEC) (Murase et al., 2012). Shiga toxin-producing E. coli (STEC) are known to produce Shiga toxins (Stx1, Stx2) and are recognized as the causative agent for life-threatening conditions in humans, such as hemorrhagic colitis and/or hemolytic-uremic syndrome (HUS). While Enterohemorrhagic E. coli (EHEC) produces the plasmid-encoded Enterohaemolysin (hlyA). Hence, strains that carry both Stx and hlyA virulence genes are potentially considered more dangerous to humans than those with only one virulence gene (Rasheed et al., 2014).
In this study, the findings confirmed the presence of different encoding virulence genes in E. coli strains isolated from different samples at a rate of 66.6% (8 out of 12). Markedly, most of the E. coli strains, isolated from eggshells of eggs produced from cage housing systems, encoded for virulence genes (6 out of 8), followed by an ovarian sample (1 out of 8) and a fecal sample (1 out of 8). The most predominant virulence gene among the positive E. coli isolates was the stx gene. This result agreed with (Morran et al., 2013), who found that 53% of the E. coli strains isolated from chickens’ lesions contained stx gene sequences, with the majority containing the stx1 allele, but no eae or E-hlyA virulence genes were detected in stx-positive strains. In our study, the virulence genes stx1, eae, hlyA, and stx2 were identified from a fecal sample from the same flocks raised in cage systems, which suggests the possibility of fecal contamination of the corresponding eggshells. Similarly, AL-Ashmawy (2013) in her study reported the detection of the stx2 virulence gene in 37/39 of the E. coli strains isolated from table eggs. On the other hand, the results of the current study differed from a study done in Egypt by (Galal et al., 2013), who detected both stx1 and stx2 genes with the eaeA gene in 2/19 (10.52%) of egg samples. Furthermore, AL Ashmawy, (2013) reported either stx1 or stx2 with eaeA gene in 3/19 (15.78%) of egg samples.
Staphylococcus aureus MecA virulence gene conferred resistance to methicillin and other β-lactam antibiotics, by altering the penicillin-binding protein located within the cell wall. This alteration renders antibiotics that act by interfering with bacterial cell wall synthesis (Sexton et al., 2006). Uncontrolled use of antibiotics in livestock production may be responsible for the emergence of antibiotic resistance in S. aureus strains, which may cause infections to human handlers (Price et al., 2012). The results revealed that Staphylococcus aureus was detected in 11 fresh and stored egg composites collected from deep litter houses. Out of the 11 Staphylococcus aureus isolates, 45% (5/11) were positive for the mecA virulence gene (MRSA, methicillin-resistant S. aureus). These results agreed with (Eid et al., 2015), who reported that four isolates out of the 11 tested for Staphylococci were positive for the mecA gene. However, (Pyzik et al., 2014) detected the mecA gene in two S. aureus-like strains isolated from table eggs. According to the guidelines of Egyptian Organization for Standardization and Quality Control, table egg content should be free from Staphylococcus aureus (Nil) (EL-Kholy et al., 2020).
Collectively, these results suggest that housing type does influence the microbial quality of table eggs. Specifically, our results suggest that eggs produced from cage houses have better microbial quality than eggs produced from deep litter houses. Moreover, our finding could be evidence for the probability of the transfer of the antimicrobial resistance from food-producing animals to humans, either directly via the food chain or via the close contact between infected animals and humans.
CONCLUSION
Eggs are considered a valuable nutritive source; however, they could be a potential source of illness and food poisoning to humans. The environment in which the eggs are produced, handled, and stored does influence the quality and safety of table eggs. Eggs produced from the cage housing system were much cleaner than those produced from the deep litter housing system. E. coli and Staphylococcus aureus strains encoding virulence genes of public health importance could contaminate the external or internal content of eggs, inducing illness in consumers. Regular monitoring of internal and external quality parameters of table eggs should be implemented. Storage of egg till marketing should be in low temperatures to save the eggs from deterioration and microbial contamination.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
Funding
No funding was supporting this work.
AUTHORS CONTRIBUTION
All authors shared in the aim of the work and designing of the study; Mahmoud A.M. Khalf: Farm visits, Collection of egg and environmental samples and technical data; Karima M. Fahim and Elshaimaa Ismael: Egg quality measurements and microbiological analysis; Sara Nader: Molecular analysis of bacterial isolates; Elshaimaa Ismael: Data analysis and interpretation. All authors shared in writing and revising the manuscript.
REFERENCES






