Advances in Animal and Veterinary Sciences
Research Article
In-vitro Antimicrobial Activity and In-vivo Prophylactic Influence of Tulathromycin against Respiratory Diseases in Dairy Heifers Exposed to Cold Stress
Essam S. Soliman1*, Ahmed E. Mahmoud2
1Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; 2Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Abstract | Calf health was prioritized as one of the most important issues facing the dairy industry. The in-vitro antimicrobial and the in-vivo prophylactic actions of tulathromycin in dairy heifers exposed to harsh macroclimate concerning hematological, biochemical, and bacteriological profiles were evaluated. Tulathromycin 15, 20, and 25* mg were tested against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida (1.8 × 108 CFU/ml), and E. coli O6 (3.5 × 109 CFU/ml) at 0.25, 0.5, 1.0, and 2.0 h using minimal inhibitory concentration procedures. Experimental 30 dairy heifers with an average body weight of 30 kg were divided into two groups, the first was injected with 2.5 ml tulathromycin 25* mg, and the second was kept as control. A total number of 690 samples including 240 in-vitro samples (bacterial- tulathromycin mixes) and 450 in-vivo samples (150 whole blood samples, 150 sera, and 150 nasal swabs) were collected.The in-vitro study revealed that tulathromycin 25* mg achieved highly significant (P<0.01) efficiency with 100% killing against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 at 1.0, 1.0, 0.5, 0.5, and 0.5 h, respectively. In-vivo measured hematological and biochemical parameters revealed no significant differences between the injected and control heifers with significant (P≤0.05) declines in alanine aminotransferase and triglycerides, as well as, significant (P≤0.05) increases in total cholesterol at the 4th-week post-injection. The bacteriological assessments revealed highly significant (P<0.01) declines of total bacterial, Enterobacteriaceae, Streptococcus, Haemophilus, and Pasteurella counts in injected dairy heifers compared to the control. Temperature and humidity revealed non-significant weak correlations with hematological and biochemical parameters. The study concluded an efficient in-vitro antimicrobial, as well as, protective and preventive in-vivo activities of tulathromycin without any modifications in the hematological and biochemical parameters of dairy heifers exposed to cold macroclimatic conditions.
Keywords | Antimicrobial, Cold Stress, Dairy Heifers, Macroclimate, Tulathromycin, Preventive.
Received | March 31, 2021; Accepted | May 06, 2021; Published | July 01, 2021
*Correspondence | Essam S Soliman, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; Email: soliman.essam@vet.suez.edu.eg
Citation | Soliman ES, Mahmoud AE (2021). In-vitro antimicrobial activity and in-vivo prophylactic influence of tulathromycin against respiratory diseases in dairy heifers exposed to cold stress. Adv. Anim. Vet. Sci. 9(8): 1211-1222.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1211.1222
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Soliman and Mahmoud. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The growth, performance, and survival of newly born dairy heifers depend on their ability to withstand and endure the impact of the harsh surrounding macroclimatic conditions concerning wet and cold weather during winter and early spring (Van De Stroet et al., 2016). These harsh macroclimatic circumstances might present negative impacts on heifer’s welfare, growth, and survivability (Chester-Jones et al., 2017), as well as, contribute to hypothermia in heifers. The modern biosecurity programs aim to reduce the influence of cold stress on heifers through efficient hygienic practices and strict preventive programs (Pineda et al., 2016). The preventive measures taken included a proper housing system with a suitable and sanitary parlor, good bedding with high insulation degrees, a proper heating system, sufficient food supply, proper disinfection procedures, rodent control, fly proof, efficient management strategies, proper handling of waste and manure, and hygienic carcasses disposal (Ghasemi et al., 2017).
Good management practice encourages heifers to resist the cold harsh macroclimatic conditions through several actions including shivering and increase the basal metabolic rates to increase the rate of thermogenesis and reduce the rate of thermolysis (Cannon and Nedergaard 2011). Once a dairy heifer is overwhelmed by the macroclimatic cold stress, she will be more susceptible to disease concerning the respiratory system (Borderas et al., 2009; Drackley, 2008; Lago et al., 2006). Bovine respiratory diseases (BRDs) considered the most common diseases affecting back-grounding and feedlot cattle as they caused higher losses as a result of poor performance and deaths (Hulbert and Moisá, 2016; Butler et al., 2006).
Tulathromycin is a macrolide antibiotic that is relatively safe and highly effective in the field of prophylaxis and control of bovine diseases caused by Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis in cattle, as well as, against infectious diseases in high-risk calves (Murray et al., 2016). Tulathromycin has been used for treating bovine footrot caused by Fusobacterium necrophorum and Porphyromonas levii (Papich 2016). Tulathromycin once administered and absorbed, can concentrate in the cytoplasm of the white blood cells and enhance its effectiveness against intracellular micro-organisms (Nowakowski et al., 2004). Tulathromycin 25* mg/ml (Recommended by the manufacturer) at a single dose of 2.5 ml is rapidly absorbed, widely distributed, and achieved higher concentrations in the lung for long periods (Nutsch et al., 2005; Skogerboe et al., 2005; Rooney et al., 2005).
The current study investigated the in-vitro antimicrobial activity of different concentrations of tulathromycin (15, 20, and 25* mg/ml) against Streptococcus pneumonie (1.8 × 108 CFU/ml), Streptococcus pyogenes (1.8 × 108 CFU/ml), Haemophilus influenzae (1.8 × 108 CFU/ml), Pasteurella multocida (1.8 × 108 CFU/ml), and E. coli O6 (3.5 × 109 CFU/ml), as well as, the preventive and prophylactic influences of tulathromycin 25* mg in dairy heifers exposed to adverse cold weather concerning its impact on some hematological parameters, biochemical parameters, and nasal microbiota.
MATERIALS AND METHODS
The materials, methodology, and study design were approved by the Scientific Research Ethics Committee on animal and poultry researches, Faculty of Veterinary Medicine, Suez Canal University, Egypt with approval number (2021001).
In-vitro evaluation of tulathromycin
Tulathromycin: Tulathromycin 25 mg (Zoetis®) injectable solution was purchased from a veterinary clinic, Ismailia Governorate, Egypt. The tulathromycin solution was subjected to serial dilutions using distilled water to produce final concentrations of 15, 20, and 25* mg (recommended by the manufacturer) into 50 ml capacity Falcon tubes and held at 4°C until testing.
Microbial cultures and propagation
Streptococcus pneumonie (Thermo ScientificTM Culti-LoopsTM Streptococcus pneumoniae ATCCTM 49619TM), Streptococcus pyogenes (Thermo ScientificTM Culti-LoopsTM Streptococcus pyogenes ATCCTM 12384TM), Haemophilus influenzae (Thermo ScientificTM Culti-LoopsTM Haemophilus influenzae ATCCTM 35540TM), Pasteurella multocida (MicrobiologiscTM Pasteurella multocida ATCCTM 12945TM), and E. coli O6 suspension (2.5 × 105 CFU/ml) were purchased from Animal Health Research Institute (AHRI), Cairo, Egypt.
Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, and Pasteurella multocida culti-loops were propagated as recommended by Herigstad et al. (2001) into tryptone soy broth (Thermo Scientific™ Oxoid™ Tryptone Soya Broth, CM0129, 500 g) at 37°C for 24 hours. Ten microliters were transferred aseptically from Streptococcus pneumonie and Streptococcus pyogenes tubes onto K-F Streptococcus agar (Thermo Scientific™ Oxoid™ K-F Streptococcus Agar, CM0701B, 500 g), and from Haemophilus influenza and Pasteurella multocida tubes onto Columbia blood agar with 5% sheep blood (Thermo Scientific™ Oxoid™ Columbia Blood Agar Base, CM0331, 500 g) at 37°C for 24 hours. The typical colonies were counted, picked up, and resuscitated in buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500 g) to obtain a suspension of 1.8 × 108 CFU/ml for each microorganism.
E. coli O6 suspension was propagated as recommended by Soliman et al. (2018) into Mac-Conkey broth (Thermo Scientific™ Oxoid™ MacConkey Broth, CM0505, 500 g) at 42°C for 24 hours. Ten microliters were transferred aseptically onto eosine methylene blue agar (EMB, Modified Levine Eosine Methylene Blue Thermo ScientificTM OxoidTM, CM0069B, 500 g) at 37°C for 24 hours. The typical colonies were counted, picked up, and resuscitated in buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500 g) to obtain a suspension of 3.5 × 109 CFU/ml.
In-vitro antimicrobial activity of tulathromycin
The in-vitro evaluation of tulathromycin antimicrobial activity was carried out using minimal inhibitory concentration (MIC) procedures according to Soliman et al. (2016). One ml from Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 suspensions were added to four replicates 9 ml of each of the tulathromycin different concentrations (15, 20, 25* mg/ml), and mixed thoroughly using vortex (Vortex Mixer XH-D, 2800r/m, 30 W, Bowel and disk shapes). After 0.25, 0.5, 1.0, and 2.0 h contact times, one ml was transferred from each mix, added to 9 ml physiological saline resuscitation tubes held previously at 4°C, and mixed thoroughly using the vortex. The tubes were transferred for the bacteriological assessment.
In-vivo evaluation of tulathromycin
Study area and time: The study was carried out in a private sector dairy farm located in El-Sharkia Governorate, Egypt. The farm was located at coordinates 30°44′28″N 32°00′23″E. The experimental study was carried out through the 2nd week of January to the end of the 3rd week of February 2020.
The dairy farm was designed as a milking housing system that was composed of a loose housing system associated with non-shaded yards and a herringbone milking parlor. The farm was supported with isolation pens for the suspected and diseased animals in the southern part of the farm. The drainage system was based on a dirty floor system with weekly scratching and removal of the top 5 cm monthly to discourage the anaerobic conditions.
The claves were reared in an artificial outdoor rearing system in which the calves are separated from their dams after six hours of birth to allow them to suck colostrum. Each calf was housed in a separate pen for two weeks then removed to a collective alternative hutch system to facilitate the cleaning and scratching of the dirty floor. Calves were feed on milk substitutes twice daily in buckets at a distance from the floor to encourage the passage of the milk to the abomasum directly via the esophageal groove.
The floors were disinfected from time to time using slaked lime in the presence and/or absence of animals without stimulating the dustiness to minimize the development of respiratory diseases.
Experimental animals and treatment
Thirty (30) dairy heifers with an average body weight of 30 kg were selected at the time of birth. Heifers were divided into two groups (15 heifers each, 3 replicates of five heifers). The heifers of the first group (G1) were injected with 2.5 ml tulathromycin 25mg*/ml subcutaneous (SC) at birth and the second group (G2) was used as control. Dairy heifers were monitored for four weeks post-injection for the general health status and the development of any respiratory manifestations.
The macroclimatic minimum and maximum temperature and relative humidity were recorded during each sampling time using thermometers (ThermoPro® TP50 Digital LCD Thermometer Hygrometer Temperature Humidity Meter) and Thermohygrometer (Digital Thermometer Hygrometer Indoor Outdoor Temperature Meter Humidity Monitor with LCD Alarm Clock, 3M Probe Cord).
Sampling
A total number of 690 samples including 240 in-vitro bacterial- tulathromycin mixes (3 concentrations × 4 replicates × 5 microbial cultures × 4 contact times) and 450 in-vivo samples (150 whole blood samples, 150 sera, and 150 nasal swabs) were collected during the study. The whole blood samples, sera, and nasal swabs samples were collected at the injection time (zero time), one-week post-injection (P1), two weeks post-injection (P2), three weeks post-injection (P3), and four weeks post-injection (P4). All samples were preserved in a dry ice-box and transferred to the laboratory for analysis.
Whole blood samples were collected on sterile ready to use vacutainer tubes (VACUETTE® TUBE 5 ml K3E K3EDTA 13x100 lavender cap-black ring, PREMIUM), mixed thoroughly, and transferred to the laboratory for immediate hematological examination. Sera samples were collected on serum vacutainers (BD Vacutainer® Serum tubes, 10.0mL, 16 x 100mm, Plastic, Additive: Clot Activator, Silicone Coated, Red Conventional Closure, and Paper Label), centrifuged (Fisher®Thermo Scientific CL10 Centrifuge w/ F-G3 Rotor with a max RPM of 4000) at 3000 rpm for 10-15 min, clear non-hemolyzed sera were pipetted using an automatic pipette (Thermo Scientific™ Finnpipette™ Adjustable Volume Single-Channel Micro Pipettor, 100 to 1000 μL microliter Volume) into Eppendorf tubes, and stored at -20°C until biochemical examination. Nasal swabs were collected on 9 ml buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500g), transferred to the laboratory, and preserved at 4°C until bacteriological assessment (Soliman et al., 2017).
Hematological and biochemical profile
The whole blood samples (150 samples, 5 samples per animal, one per each sampling time) were examined for red blood cells count (RBCs, ×106/µl), the white blood cells count (WBCs, ×103/µl), hemoglobin concentrations (Hb expressed as g/dl), platelet counts (×103/µl), mean corpuscular hemoglobin concentrations (MCHC expressed as g/dl) using Sysmex XP-300 Automated Hematology Analyzer. Sera (150 samples, 5 samples per animal, one per each sampling time) were examined for total protein (TP expressed as g/dl), alanine aminotransferase (ALT expressed as IU/L), creatinine (CREAT expressed as mg/dl), glucose (GLUCO expressed as mg/dl), triglycerides (TG expressed as mg/dl), and total cholesterol (TC expressed as mg/dl) using Chemical Analyzer Semi-auto Photometer 5010 (Germany).
Bacteriological examination
Nasal swabs on buffered peptone water (150 samples, 5 samples per animal, one per each sampling time) and bacterial- tulathromycin mixes (240 samples, 3 concentrations × 4 replicates × 5 microbial cultures × 4 contact times) were prepared by ten-fold serial dilutions up to 107 to cover all the chances of the microbial growth from the samples as recommended by American Public Health Association; APHA, (2017).
Total bacterial counts onto Standard Plate count agar (SPA, Thermo Scientific™ Oxoid™ Plate Count Agar, CM0325, 500 g), Enterobacteriaceae counts onto eosine methylene blue agar (EMB, Modified Levine Eosine Methylene Blue Thermo ScientificTM OxoidTM, CM0069B, 500 g), Streptococcus counts onto K-F Streptococcus agar (Thermo Scientific™ Oxoid™ K-F Streptococcus Agar, CM0701B, 500 g), and Haemophilus and Pasteurella counts onto Columbia blood agar with 5% sheep blood (Thermo Scientific™ Oxoid™ Columbia Blood Agar Base, CM0331, 500 g) were carried out at 37°C for 24-36 hours. The microbial counts were conducted using the drop plate technique as recommended by Kim and Lee, (2016). The plates were counted as recommended by Murray et al. (2015) using dark filed colony counter (R164109 Reichert-Jung Quebec Darkfield 3325 Colony Counter).
Statistical analysis
The statistical analysis was carried out using the statistical package for social sciences (SPSS version 21, IBM, SPSS Inc., USA; SPSS, 2016). The data were analyzed using multifactorial analysis of variance (two-tailed ANOVA) to investigate the in-vitro antimicrobial activity of tulathromycin different concentrations (15, 20, and 25* mg/ml) against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenza, Pasteurella multocida, and E. coli O6 concerning different exposure times (0.25, 0.5, 1.0, and 2.0 h), as well as, the prophylactic influence of tulathromycin on hematological, biochemical, and bacteriological parameters concerning different sampling times (zero-time, P1, P2, P3, and P4). The statistical model was summarized as follow:
Yijk= µ + αi + βj + (αβ)ij + Ɛijk
Where Yijk was the measurement of dependent variables; µ was overall mean; αj was the fixed effect of the tulathromycin, βj was the fixed effect of the exposure times or sampling times, (αβ)ij was the interactions, and Ɛijk was the random error. Pearson’s correlation (r) was calculated to determine the correlation coefficient between macroclimatic conditions like temperature and relative humidity with the bacterial counts, measured bacterial, and hematological parameters. The bacterial counts were transformed and expressed as logarithmic counts (Log10) using Microsoft Excel 2016. The significance was expressed as highly significant at (P<0.01), significant at (P≤0.05), and non-significant at (P>0.05).
RESULTS
In-vitro antimicrobial activity of tulathromycin
The overall means in Table-1 revealed highly significant (P<0.01) efficiency of tulathromycin 25* mg (recommended by the manufacturer) against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 compared with the other tested concentrations (15 and 20 mg/ml). The overall means concerning the exposure times (Table-1) revealed highly significant (P<0.01) increases in the killing percentages with the increased exposure times in all tested concentrations against all microorganisms.
Tulathromycin 15 mg/ml achieved highly significant (P<0.01, Table-1) efficiency with 34.3, 36.0, 28.0, 27.4, and 72.8% killing against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6, respectively at 2.0 h. Tulathromycin 20 mg/ml in Table-1 achieved highly significant (P<0.01) efficiency with 62.4, 64.2, 53.3, 48.0, and 100.0% killing against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6, respectively at 2.0 h. Meanwhile, tulathromycin 25* mg/ml achieved highly significant (P<0.01, Table-1) efficiency with a 100% killing percentage against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 at 1.0, 1.0, 0.5, 0.5, and 0.5 h, respectively.
Clinical examinations
The dairy heifers injected with tulathromycin revealed neither abnormalities nor development of any clinical manifestations during the study period. On the other hand, 40% of the control animals under study exhibited pneumonia
Table 1: In-vitro antimicrobial activity (killing % ±SE) of tulathromycin concentrations against different microorganisms via minimal inhibitory concentration test.
| Conc. |
Contact times |
Strep pneumonie | Strep pyogenes | Haemoph influenzae | Past multocida |
E. coli O6 |
| Overall means concerning tulathromycin concentrations | ||||||
| 15 mg |
26.3c±0.046 |
24.2c±0.062 |
16.7c±0.084 |
18.7c±0.066 |
47.4c±0.075 |
|
| 20 mg |
43.8b±0.059 |
50.2b±0.039 |
39.8b±0.078 |
36.3b±0.020 |
73.9b±0.057 |
|
| 25 mg* |
82.2a±0.079 |
88.3a±0.038 |
96.3a±0.018 |
96.3a±0.005 |
88.3a±0.077 |
|
| P-value | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | |
| Overall means concerning exposure times | ||||||
| 0.25 h |
34.2d±0.086 |
37.6d±0.024 |
41.2d±0.036 |
39.1d±0.061 |
36.8d±0.001 |
|
| 0.5 h |
45.1c±0.026 |
49.9c±0.064 |
49.6c±0.071 |
51.0c±0.054 |
69.1c±0.002 |
|
| 1.0 h |
58.2b±0.002 |
62.3b±0.024 |
52.6b±0.038 |
53.2b±0.092 |
82.8b±0.094 |
|
| 2.0 h |
65.5a±0.052 |
66.7a±0.025 |
60.4a±0.054 |
58.5a±0.092 |
90.9a±0.053 |
|
| P-value | 0.000 | 0.000 | 0.002 | 0.001 | 0.00 | |
| Tulathromycin concentrations by exposure times interactions | ||||||
| 15 mg | 0.25 h |
12.7d±0.011 |
14.0d±0.037 |
10.1d±0.011 |
12.8d±0.066 |
12.9d±0.012 |
| 0.5 h |
27.8c±0.033 |
17.2c±0.027 |
12.3c±0.017 |
15.5c±0.048 |
43.9c±0.060 |
|
| 1.0 h |
30.2b±0.032 |
29.5b±0.020 |
16.3b±0.038 |
19.1b±0.039 |
60.0b±0.079 |
|
| 2.0 h |
34.3a±0.071 |
36.0a±0.016 |
28.0a±0.020 |
27.4a±0.018 |
72.8a±0.067 |
|
| 20 mg | 0.25 h |
30.2d±0.032 |
29.5d±0.020 |
28.0d±0.020 |
19.1d±0.039 |
44.0d±0.030 |
| 0.5 h |
38.1c±0.012 |
48.6c±0.045 |
36.5c±0.045 |
37.4c±0.058 |
63.3c±0.020 |
|
| 1.0 h |
44.3b±0.078 |
57.6b±0.039 |
41.5b±0.027 |
40.4b±0.004 |
88.4b±0.052 |
|
| 2.0 h |
62.4a±0.021 |
64.2a±0.033 |
53.3a±0.053 |
48.0a±0.023 |
100.0a±0.000 |
|
| 25 mg* | 0.25 h |
59.5c±0.054 |
69.3c±0.092 |
85.4b±0.021 |
85.4b±0.021 |
53.4b±0.028 |
| 0.5 h |
69.3b±0.092 |
83.8b±0.042 |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
|
| 1.0 h |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
|
| 2.0 h |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
100.0a±0.000 |
|
| P-value | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
Strep=Streptococcus, Haemoph= Haemophilus, Past=Pasteurella, E. coli=Escherichia coli, SE=Standard error.
Table 2: Hematological parameters (Mean ±SE) in dairy heifers exposed to cold stress conditions.
| Groups | Time | Hb (g / dl) |
RBCs (×106 / µl) |
MCHC (g / dl) |
WBCs (×103 / µl) |
Platelets (×103 / µl) |
| Overall means among groups | ||||||
| G1 |
7.6a±0.012 |
6.00a±0.017 |
38.0a±0.002 |
6.61a±0.631 |
249a±0.076 |
|
| Gc |
7.5a±0.011 |
6.01a±0.015 |
32.3b±0.001 |
6.64a±0.023 |
264a±0.055 |
|
| P-value | 0.641 | 0.930 | 0.000 | 0.921 | 0.010 | |
| Overall means among sampling times | ||||||
| Zero |
8.0a±0.022 |
6.14a±0.001 |
33.0c±0.021 |
6.98a±0.033 |
261a±0.044 |
|
|
1st wk |
8.0a±0.032 |
6.12a±0.009 |
34.0bc±0.001 |
7.03a±0.061 |
259a±0.024 |
|
|
2nd wk |
7.8a±0.021 |
6.27a±0.011 |
34.0bc±0.002 |
6.82a±0.045 |
246a±0.011 |
|
|
3rd wk |
7.0b±0.011 |
6.02a±0.002 |
36.8ab±0.002 |
6.45ab±0.014 |
262a±0.001 |
|
|
4th wk |
6.8b±0.001 |
5.50b±0.002 |
37.9a±0.002 |
5.82b±0.055 |
254a±0.040 |
|
| P-value | 0.000 | 0.001 | 0.025 | 0.062 | 0.378 | |
| Treatment by sampling times interactions | ||||||
| G1 | Zero |
8.0a±0.003 |
6.10a±0.008 |
32.9b±0.026 |
6.80a±0.074 |
245a±0.013 |
|
1st wk |
8.2a±0.002 |
6.14a±0.002 |
35.4b±0.017 |
7.11a±0.049 |
266a±0.016 |
|
|
2nd wk |
7.7a±0.001 |
6.12a±0.001 |
35.7b±0.022 |
6.68a±0.021 |
229b±0.021 |
|
|
3rd wk |
6.9b±0.031 |
6.28a±0.002 |
41.8a±0.022 |
6.90a±0.023 |
249a±0.004 |
|
|
4th wk |
7.0a±0.014 |
5.38b±0.006 |
44.1a±0.001 |
5.54b±0.068 |
255a±0.003 |
|
| G2 | Zero |
8.0a±0.001 |
6.18a±0.006 |
33.2a±0.026 |
7.16a±0.068 |
277a±0.001 |
|
1st wk |
7.9a±0.002 |
6.09a±0.001 |
32.6a±0.026 |
6.96a±0.071 |
251a±0.006 |
|
|
2nd wk |
7.8a±0.001 |
6.41a±0.004 |
32.4a±0.021 |
6.96a±0.044 |
262a±0.033 |
|
|
3rd wk |
7.2a±0.006 |
5.76b±0.006 |
31.8a±0.022 |
6.13a±0.042 |
275a±0.002 |
|
|
4th wk |
6.5b±0.004 |
5.63b±0.004 |
31.6a±0.017 |
6.10a±0.086 |
253a±0.001 |
|
| P-value | 0.726 | 0.115 | 0.001 | 0.561 | 0.029 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
G1=Tulathromycin injected group, Gc=Control group, Hb=Hemoglobin, RBCs=Red blood cells, MCHC=Mean corpuscular hemoglobin concentration, WBCs=White blood cells, SE=Standard error.
Table 3: Biochemical parameters (Mean ±SE) in dairy heifers exposed to cold stress conditions.
| Gs | Time |
TP g/dl |
ALT IU/L |
CREAT mg/dl |
GLUCO mg/dl |
TG mg/dl |
TC mg/dl |
| Overall means among groups | |||||||
| G1 |
5.7a±0.014 |
3.0a±0.022 |
0.9a±0.027 |
89a±0.032 |
110a±0.052 |
57a±0.098 |
|
| Gc |
5.8a±0.016 |
2.6a±0.013 |
0.9a±0.029 |
78a±0.080 |
100a±0.087 |
57a±0.015 |
|
| P-value | 0.672 | 0.098 | 0.757 | 0.072 | 0.015 | 0.985 | |
| Overall means among sampling times | |||||||
| Zero |
5.4a±0.033 |
3.1a±0.029 |
1.0a±0.061 |
93a±0.074 |
118a±0.078 |
48b±0.025 |
|
|
1st w |
5.9a±0.029 |
3.4a±0.030 |
1.0a±0.049 |
87a±0.075 |
105ab±0.073 |
48b±0.051 |
|
|
2nd w |
5.9a±0.022 |
2.5ab±0.016 |
0.9a±0.034 |
86a±0.052 |
99b±0.099 |
47b±0.077 |
|
|
3rd w |
5.8a±0.018 |
3.0a±0.043 |
0.9a±0.039 |
73a±0.096 |
104ab±0.023 |
69a±0.079 |
|
|
4th w |
5.8a±0.013 |
2.1b±0.011 |
0.9a±0.032 |
81a±0.061 |
99b±0.059 |
71a±0.089 |
|
| P-value | 0.470 | 0.013 | 0.161 | 0.334 | 0.055 | 0.000 | |
| Treatment by sampling times interactions | |||||||
| G1 | Zero |
5.5a±0.042 |
3.2a±0.042 |
1.1a±0.089 |
104a±0.073 |
124a±0.015 |
54c±0.036 |
|
1st w |
5.9a±0.046 |
3.4a±0.043 |
0.9a±0.059 |
90a±0.083 |
121a±0.047 |
48c±0.087 |
|
|
2nd w |
6.0a±0.026 |
2.7ab±0.025 |
1.0a±0.038 |
99a±0.086 |
98b±0.021 |
39d±0.070 |
|
|
3rd w |
5.8a±0.026 |
3.7a±0.082 |
0.9a±0.048 |
72a±0.097 |
104b±0.018 |
63b±0.040 |
|
|
4th w |
5.4a±0.013 |
2.1b±0.019 |
0.9a±0.042 |
83a±0.076 |
105b±0.005 |
81a±0.054 |
|
| G2 | Zero |
5.2a±0.052 |
2.9a±0.040 |
0.9a±0.077 |
82a±0.049 |
111a±0.071 |
43b±0.054 |
|
1st w |
6.0a±0.036 |
3.4a±0.043 |
1.1a±0.074 |
84a±0.059 |
89b±0.05 |
49b±0.051 |
|
|
2nd w |
5.8a±0.037 |
2.4ab±0.021 |
0.9a±0.053 |
73a±0.065 |
100a±0.097 |
55ab±0.082 |
|
|
3rd w |
5.8a±0.027 |
2.3b±0.011 |
0.9a±0.063 |
74a±0.022 |
105a±0.086 |
76a±0.090 |
|
|
4th w |
6.2a±0.020 |
2.1b±0.011 |
0.9a±0.049 |
78a±0.012 |
93ab±0.081 |
62a±0.091 |
|
| P-value | 0.567 | 0.363 | 0.024 | 0.545 | 0.099 | 0.023 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01). Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
TP=Total protein, ALT=Alanine aminotransferase, CREAT=Creatinine, GLUCO=Glucose, TG=Triglycerides, TC=Total cholesterol, Gs=Groups, SE=Standard error.
Table 4: Temperature and relative humidity correlation coefficient with hematological parameters in dairy heifers exposed to cold stress conditions.
| r | Temp | Hb | RBCs | MCHC | WBCs | Plat |
| RH | 1 | -0.056 | -0.108 | -0.039 | 0.131 | -0.088 |
| HB | -0.107 | 1 | 0.647** | -0.101 | 0.089 | 0.066 |
| RBCs | -0.080 | 0.647** | 1 | -0.131 | 0.059 | 0.049 |
| MCHC | -0.059 | -0.101 | -0.131 | 1 | -0.133 | -0.014 |
| WBCs | 0.132 | 0.089 | 0.059 | -0.133 | 1 | -0.031 |
| Platelets | -0.142 | 0.066 | 0.049 | -0.014 | -0.031 | 1 |
**. Correlation is highly significant (P < 0.01). *. Correlation is significant (P < 0.05). NS. Correlation is non-significant (P < 0.05).
r= Person’s correlation coefficient, Temp=Temperature, RH=Relative humidity, Hb=Hemoglobin, RBCs, RBCs=Red blood cells, MCHC=Mean corpuscular hemoglobin concentration, WBCs=White blood cells.
Table 5: Temperature and relative humidity correlation coefficient with biochemical parameters in dairy heifers exposed to cold stress conditions.
| r | Temp | TP | ALT | CREAT | GLUCO | TG | TC |
| RH | 1 | 0.059 | 0.004 | -0.061 | 0.013 | -0.026 | 0.039 |
| TP | 0.105 | 1 | -0.017 | 0.051 | -0.126 | -0.041 | 0.158 |
| ALT | -0.075 | -0.017 | 1 | -0.078 | 0.237** | 0.338** | -0.266** |
| CREAT | -0.044 | 0.051 |
-0.078 |
1 | 0.100 | -0.115 | -0.009 |
| GLUCO | -0.008 | -0.126 | 0.237** | 0.100 | 1 | -0.003 | -0.203* |
| TG | -0.083 | -0.041 | 0.338** | -0.115 | -0.003 | 1 | -0.021 |
| TC | -0.008 | 0.158 | -0.226** | -0.009 | -0.203* | -0.021 | 1 |
**. Correlation is highly significant (P < 0.01). *. Correlation is significant (P < 0.05). NS. Correlation is non-significant (P < 0.05).
r= Person’s correlation coefficient, Temp=Temperature, RH=Relative humidity, TP=Total protein, ALT=Alanine aminotransferase, CREAT=Creatinine, GLUCO=Glucose, TG=Triglycerides, TC=Total cholesterol.
like manifestations including sneezing, coughing, serous nasal discharges, hyperthermia, reduced feed intake, and the dull sound of the lung on auscultation.
Hematological examinations
The overall means in Table-2 revealed no significant differences in all the measured hematological parameters between the injected and control heifers.
On a time scale, red blood cells and platelet counts revealed no significant differences in Table-2 between the injected and control heifers. While, hemoglobin and white blood cells (Table-2) revealed highly significant (P<0.01) increases at the zero, 1st, and 2nd weeks post-injection compared with the rest of sampling times during the study period with no significant differences between the three weeks. Mean corpuscular hemoglobin concentrations revealed in Table-2 highly significant (P<0.01) increases at the 4th week post-injection compared with all other monitoring times.
Hemoglobin, red blood cells, white blood cells, and platelets (Table-2) revealed highly significant (P<0.01) declines only at the 3rd, 4th, 4th, and 2nd-week post-injection in the injected animals with no significant differences between the values at the other sampling times. Mean corpuscular hemoglobin concentrations in Table-2 revealed highly significant (P<0.01) increases at the 3rd and 4th weeks post-injection.
Biochemical examinations
The overall means revealed no significant differences (Table-3) in all the measured parameters between the injected and control calves.
Total protein, creatinine, and glucose revealed in Table-3 no significant differences in all animals under study. Alanine aminotransferase and triglycerides revealed significant (P≤0.05) declines as time passes on injected animals compared to the control. Total cholesterol, on the other hand, revealed significant (P≤0.05) increases at the 4th-week post-injection in the injected animals compared to the control.
Macroclimatic conditions
Temperature and relative humidity (Table-4) revealed weak negative non-significant correlations with hemoglobin, red blood cells, mean corpuscular hemoglobin concentrations, and platelet counts, as well as, weak positive non-significant correlations with white blood cells.
The temperature in Table-5 revealed weak positive non-significant correlations with total protein, alanine aminotransferase, glucose, and total cholesterol, as well as, weak negative non-significant correlations with creatinine and triglycerides. Relative humidity (Table-5) revealed weak positive non-significant correlations with total protein and weak negative non-significant correlations with alanine aminotransferase, creatinine, glucose, triglycerides, and total cholesterol.
Bacteriological examinations
The overall means revealed in Figure-1A highly significant (P<0.01) declines of total bacterial, Enterobacteriaceae, Streptococcus, Haemophilus, and Pasteurella counts in injected dairy calves compared to the control animals. The measured bacterial counts revealed (Figure-1B) highly significant (P<0.01) declines as the time of the study proceed.
The animal groups by the sampling times interactions in Figures 2A and 2B revealed highly significant (P<0.01) declines of total bacterial, Enterobacteriaceae, Streptococcus, Haemophilus, and Pasteurella counts in animals injected with tulathromycin compared to the control dairy heifers.
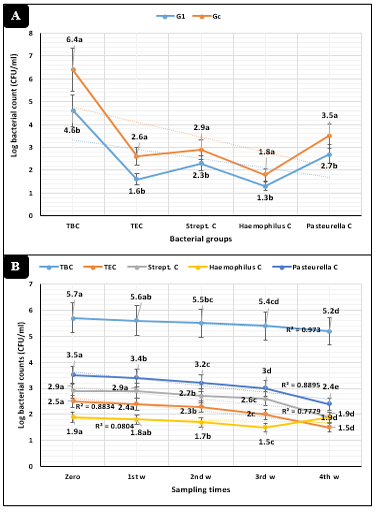
Figure 1: Bacteriological assessments (Mean ±SE) in dairy heifers exposed to cold stress. A) Concerning animal groups. B) Concerning sampling times. G1=Heifers injected with tulathromycin, Gc=Control, TBC=Total bacterial counts, TEC=Total Enterobacteriaceae counts, Strept. C=Streptococcus counts.
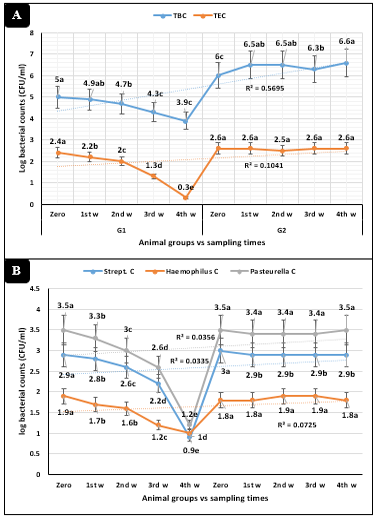
Figure 2: Microbiological parameters (Mean ±SE) in dairy heifers exposed to cold stress conditions concerning sampling times interactions. A) Total bacterial (TBC) and Enterobacteriaceae (TEC) counts. B) Streptococcus, Haemophilus, and Pasteurella counts
DISCUSSION
Newly born calves should respond properly to the surrounding environment with a certain degree of adaptation that might be difficult under certain circumstances depending on the biosecurity and management system in the dairy farm (Sjaastad et al., 2010). The dairy industry has been directed with greater extent toward the intensification, reproduction at the 2nd instead of the 3rd year of life, the early separation of the newly born calves from their dams, foster rearing, synchronization of estrus to focus calving in the cold seasons (winter and early spring), these circumstances contributed to the existence of stressful conditions on calves starting from day one (Ellingsen et al., 2015).
At the time of birth, calves exhibited higher degrees of thermolysis that are aggravated by extreme macroclimatic conditions and the evaporation action of the fetal fluids surrounding the newly born (Kirovski, 2015). Supplementing calves at the early hours of life with colostrum plays an important role in raising immunity and adjusting the physical conditions of calves (McGrath et al., 2015; Savino et al., 2011).
An emphasis on the preventive measures in dairy farms is critical, limiting the need for subsequent intervention. These preventive measures should include a complete accurate physical examination of the heifers’ bodies to confirm the physiological status of the heifers according to Silva et al. (2016) and Windeyer et al. (2014). Also, the preventive measures included the administration of some prophylactic drugs like tulathromycin that might protect the calves from adverse circumstances that might contribute to the development of some respiratory diseases as recommended by Holman et al. (2018) and Fontes Novo et al., (2015).
The current study recorded an extreme and significant in-vitro antimicrobial activity of tulathromycin against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 with a significant 100% killing percentage. The results were consistent with those recorded by Gorden and Plummer (2010) who recorded the significant influence of tulathromycin on Pasteurella multocida, Pasteurella hemolytica, and Mycoplasma dispar that have been recorded as the most common infectious causes of respiratory diseases in calves. Keith and McGuirk, (2009), and McGuirk (2008) reported that respiratory diseases might be caused by non-infectious causes like insufficient or lack of passive immunity and/or improper calve housing macroclimate.
Smith et al. (2017) revealed a significant influence of tulathromycin and enrofloxacin in 8-months-old calves against Salmonella and Campylobacter for 28 days post-treatment. Pereira et al. (2016) explained the broad-spectrum action of tulathromycin macrolides on the enteric microorganisms that might attack and contribute to diarrhea in newly born calves. Foditsch et al. (2019) and Tempini et al. (2018) recorded significant influence of tulathromycin and enrofloxacin with no significant relative gene-linked resistance in calves up to 14 days. Timsit et al. (2017) also recorded the high efficient antimicrobial activities of tulathromycin against some bacterial pathogens using the traditional cultural means and sensitivity test.
The current study recorded clinically ill animals up to 41% in the control group compared to zero% illness in the injected animals. These results were synchronized with those reported by Stanton et al. (2010) who recorded a significant reduction in the incidence of respiratory diseases up to 4.9% compared to the control animals that recorded about 46.8% during the adverse environmental conditions. They also recorded a significant decrease in morbidity up to 9.3% compared to 34.5% in the control groups with the increase in the performance and livability of the injected animals. Abell et al. (2017) recorded the metaphylactic and therapeutic actions of tulathromycin as a single or combined form and in turn, reduced the incidence of respiratory diseases in dairy animals at the time of birth.
The study revealed non-significant changes in hematological and biochemical parameters in the injected animals compared to control. The results also showed significant declines in the levels of alanine aminotransferase and triglycerides of the injected animals confirming the absence of any stresses on the animals from injection or the surrounding macroclimatic conditions. The current results were supported by those recorded by Amir et al. (2013) who revealed maintaining of the biochemical and hematological conditions in calves injected with tulathromycin. Also, Ignǎtescu et al. (2018); Soliman et al. (2020) and Soliman et al. (2021) reported that the usage of preventive and biosecurity measures like the injection of some prophylactic drugs (antibiotics as tulathromycin, prebiotics, probiotics, synbiotics, and herbal additives), proper housing design, proper building direction, maximum interior arrangement, outdoor or mixed calving system, good parlor hygienic practices, sufficient disinfection program, fly and rodent-proof, control of pet animal access to the farm, hygienic disposal of carcasses, and waste management, as well as, artificial colostrum might present solutions for the high incidence of many diseases in newly born calves with more reference to the respiratory diseases. Also, O’Connor et al. (2016) recorded significant maintaining of the sera parameters in calves with no significant differences between tulathromycin and enrofloxacin treatment. Crosby et al. (2018) revealed significant superior efficiency of tulathromycin (33.7%) over enrofloxacin (18.3%) in calves during the first 45 days of life and ensured the metaphylactic effect of tulathromycin in newborn calves.
The current study also revealed significant reductions in total bacterial, Enterobacteriaceae, Streptococcus, Haemophilus, and Pasteurella counts in the nasal swabs collected from the newly born calves. The results were consistent with these reported by Toutain et al. (2016) who found that subcutaneous injection of newborn calves with tulathromycin 2.5 mg/kg was able to significantly reduce Mannheimia haemolytica up to 66% and Pasteurella multocida up to 87%. Baptiste and Kyvsgaard, (2017) explained that tulathromycin therapy in newborn calves produced a good metaphylaxis with a significant reduction of respiratory disease incidence. Collingnon et al. (2016) recorded a significant influence of tulathromycin against Salmonella, Campylobacter, and multi-drug resistant Shigella.
Dennehy (2019) and Ferguson et al. (2018) recorded significant declines in the Escherichia coli population in the samples collected from calves treated with tulathromycin and enrofloxacin. Pereira et al. (2020) recorded that tulathromycin and enrofloxacin treatment in calves revealed a higher rate of metaphylaxis with the development of some E. coli resistant bacteria. Lin et al. (2019) revealed that extra-labeled withdrawal intervals of tulathromycin should be considered in calves when administered as a prophylactic and treatment against pneumonia. Bartram et al. (2016) also recorded high efficiency of tulathromycin against Mycoplasma bovis in calves with lower lung lesions that might be caused by Mycoplasma bovis compared with other prophylactic and treating antibiotics. Doster et al. (2018) recorded a greater prophylactic influence of tulathromycin in dairy calves against resistance fecal resistome and microbiome over the changes in geography, diet, macroclimatic exposure, and transition during the early feeding periods in the feedlot.
CONCLUSION
Tulathromycin was able to produce efficient and significant in-vitro antimicrobial activity with 100% killing efficacy against Streptococcus pneumonie, Streptococcus pyogenes, Haemophilus influenzae, Pasteurella multocida, and E. coli O6 at 1.0, 1.0, 0.5, 0.5, and 0.5 h, respectively.
Tulathromycin as a single dose was able to exhibit sufficient protective and prophylactic activities in the dairy heifers exposed to cold stress via significant reduction of the total bacterial, Enterobacteriaceae, Streptococcus, Haemophilus, and Pasteurella counts that might contribute to a higher incidence of respiratory diseases in dairy heifers under such circumstances (cold weather), as well as, maintaining the hematological and biochemical parameters in injected calves at control levels.
ACKNOWLEDGMENTS
Sincere thanking should be provided to the private sector dairy farm in El-Sharkia Governorate, Egypt for their patience and allowance to run the current experiment. Also, we sincerely thank Prof. TS Nafie and Prof. MA Sobieh for their tremendous directions during the experiment. Also, we would like to express our sincere thanking to Dr. OF Mohamed for her help in the in-vitro experiment. The current article received no specific grant from any funding agency in the public and commercial fields, and not for profit sectors.
conflict of interest
The authors declare that they have no financial or personal conflicts which may have inappropriately influenced them in writing this manuscript.
AUTHORS’ CONTRIBUTION
AEM designed the experimental design, participated in the execution of the in-vivo experiment, and writing of the manuscript. ESS conducted the in-vitro evaluation, participated in the execution of the in-vivo experiment, and writing of the manuscript.
REFERENCES






