Advances in Animal and Veterinary Sciences
Research Article
Potency of Curcumin Derived from Ethanol Extract of Turmeric (Curcuma longa L.) as the Immunostimulator in Broiler
Nurul Hidayah, Retina Yunani, Dyah Widhowati*
Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia, 60225.
Abstract | The development of the poultry industry in Indonesia has increased in recent years. However, there are several obstacles to the increasing in the poultry industry such as the diseases. In preventing of diseases transmission among the poultry, the scientist has been developed vaccine and immunostimulator. One of the prominent immunostimulators is using herbal extract such as curcumin. This study aimed to analyze the potency of curcumin as the immunostimulator in broiler. Ninety-six day-old-chicks (DOC) of broiler were used as the animal model. They were separated into four groups: I = control; II = treated with 40% curcumin; III = with 50% of curcumin; and IV = with 60% of curcumin. The treatment was conducted for seven days through the drinking water. On day 7, the chickens were vaccinated with the avian influenza vaccine. The chickens were sacrificed on day 10. The blood samples were tested against a total of heterophil and lymphocytes, the serum samples against the AI antibody, and the spleen against the proliferation of white pulp. The data were analyzed using SPSS-16. The result showed that group III and group IV has significant effects on the increasing of AI titer antibody, total of heterophil and lymphocytes, and proliferation of white pulp within the spleen tissue (p≤0.05). In conclusion, the administration of curcumin derived from turmeric extract potentially increases the immunity of broiler chickens via its ability to promote titer antibody, heterophil, lymphocytes, and proliferation of white pulp after vaccination.
Keywords | Broiler, Curcumin, Titer antibody, Heterophil, Lymphocytes.
Received | December 10, 2020; Accepted | December 29, 2020; Published | April 01, 2021
*Correspondence | Dyah Widhowati, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia, 60225; Email: dyahwidhowati@gmail.com
Citation | Hidayah N, Yunani R, Widhowati D (2021). Potency of curcumin derived from ethanol extract of turmeric (curcuma longa l.) As the immunostimulator in broiler. Adv. Anim. Vet. Sci. 9(6): 787-791.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.6.787.791
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Widhowati et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The development of the poultry industry in Indonesia has increased in recent years. However, there are several obstacles to the increasing in the poultry industry, such as diseases. Several diseases that potentially decrease the production of poultry are aflatoxicosis, Newcastle disease (ND), salmonellosis, and avian influenza (AI). AI is a prominent disease in poultry due to its high morbidity and mortality (Korteweg and Gu, 2008). In preventing of AI transmission among the poultry, the scientist has been developed vaccine and immunostimulator.
Immunostimulator is being expected to increase the cellular and immune response of the birds. Immunostimulator influence leukocytes and cells of the immune system, including splenocytes (Ali et al., 2019). Immunostimulators can be produced using natural bioactive compounds sourced from herbal. One of the prominent immunostimulators is using herbal extract, such as curcumin.
Curcumin is the derivate from turmeric’s (Curcuma longa L.) extract. Curcumin potentially inhibits the reactive oxygen species, metabolic syndrome, arthritis, and hyperlipidemia in human health (Hewlings and Kalman, 2017). In poultry, curcumin can be used as the feed additive that increases chicken’s growth performance (Abou-Elkhair et al., 2014). However, its potency in the increasing of immune response after vaccination is still complicated.
This study aims to analyze the potency of curcumin derived from the ethanol extract of turmeric (Curcuma longa L.) as the immunostimulator in broiler using several parameters, including titer antibody against AI after vaccination, heterophil and lymphocytes count, and score proliferation of white pulp in the spleen.
Material and methods
Ethic approval
This study have been approved by ethical clearance committee of Faculty of Dental Medicine, University of Airlangga, Surabaya. The animal procedure followed the guideline of animal experimentation.
Time and place
The study was conducted in January 2019 until July 2020. The study was conducted in Indonesian Institute of Sciences, Cibinong, Indonesia and Laboratory of Animal Model, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia.
Herbal species authentication
The herbal was collected from local market in Surabaya. The species of turmeric has been authenticated by Indonesian Institute of Sciences, Cibinong with registration number: 1825/IPH.1.01/If.07/VIII/2018. The certificate of authentication showed that the turmeric species was Curcuma longa L.
Curcumin standardization
The turmeric was dried and extracted using 70% ethanol following the described procedure in previous study (Prakoso and Kurniasih, 2018). Further, the extract was measured regarding its curcumin content using thin layer chromatography (TLC) (Kagan and Flythe, 2014). The TLC was conducted in three replicated. The result of TLC showed that the ethanol extract of turmeric contain 0.94±0.01 (%) of curcumin.
Research design and procedure
Ninety-six day-old-chicks (DOC) broiler strain Cobb were used as the animal model. The chickens were maintained in the Laboratory of Animal Model, Faculty of Veterinary Medicine, University of Wijaya Kusuma Surabaya, East Java, Indonesia. The chickens were maintained at 30ºC of temperature and 24-hours light schedule, however, the light intensity was reduced after 16-hours each day (Prakoso et al., 2018). They were separated into four groups, so that each group consist of 24 chickens. The group division was group I = control; II = treated with 40% curcumin; III = with 50% of curcumin; and IV = with 60% of curcumin. The doses determination refer to the previous study (Widhowati et al., 2017). The treatment was conducted for seven days through the drinking water ad libitum. On day 7, the chickens were vaccinated with the avian influenza vaccine (Medivac AI, subtype H5N1 2.3., Medion, Indonesia).
Blood collection and blood tests
The chickens were sacrificed on day 10. The blood were collected and separated into two part. The first blood sample was stored inside the blood tube with ethylenediaminetetraacetic acid (EDTA) and the second blood sample was stored inside plain tube without anticoagulant to collect its serum. The blood samples were tested against a total of heterophil and lymphocytes (Sugiharto et al., 2018), however, the serum samples were tested against the AI titer antibody. The AI titer antibody was tested using haemagglutination inhibition (HI) test following the demonstrated procedure by previous study (Zacour et al., 2016). The result of HI test was expressed as geometric mean titer (GMT).
Histopathology
After the euthanasia, the necropsied was performed and the spleen sample was collected. The spleen was cut and stored inside the 10% neutral buffer formalin. After 24 hours of fixation, the spleen was processed for histopathology using routine staining. The spleen was analyzed regarding the proliferation of white pulp by a single pathologist under blindfold condition. The histopathology was reported semi-quantitatively as a score. The scoring system was determined as follow: 10 = absent; 20 = minimal; 30 = mild; 40 = moderate; 50 = extensive. The observation was conducted on 5 area in 100× of magnification. The score of each area was summed up and it reported as the total score.
Data analysis
This study produced two types of data including numerical and categorical data. The numerical data was heterophil, lymphocytes and AI titer antibody. The categorical data was histopathology of chicken’s spleen. The numerical data was analyzed using on way ANOVA and post hoc test. In contrast, the categorical data was analyzed using Kruskal-Wallis and Man Whitney – U test. The data were measured using SPSS-16 with probability value in ≤0.05.
Results and discussion
The result showed that titer antibody of broiler chickens against AI virus significantly increase in group treated with 50% and 60% of curcumin (p≤0.05). However, group treated with 40% curcumin did not show significant differences regarding the AI titer antibody (p≥0.05). Surprisingly, the titer antibody in group treated with 50% curcumin was greater than group treated with 60% curcumin (Table 1).
Table 1: Titer antibody, heterophil and lymphocytes count of broiler chicken treated with curcumin on day 10
| Parameter | Mean ± standard deviation | |||
|
I (Control) |
II (40% curcumin) |
III (50% curcumin) |
IV (60% curcumin) |
|
| AI titer antibody | 8.57 ± 5.38 | 8.57 ± 5.38 |
52.14 ± 3.77a |
94.85 ± 18.48b |
| Heterophil | 30.57 ± 6.023 |
35.28 ± 6.10a |
37.57 ± 5.91b |
39.42 ± 2.76c |
| Lymphocytes | 65.14 ± 5.61 |
68.71 ± 3.86a |
70.14 ± 5.52b |
71.71 ± 2.98c |
a.b.cThe different superscript on the same row indicated significantly different value (p≤0.05).
In contrast, there is significantly increased regarding the number of circulatory heterophils and lymphocytes of the vaccinated chickens (p≤0.05). The increase of heterophils and lymphocytes occurred concomitantly along to the increased of curcumin concentration. The curcumin at 40% of concentration showed significant increasing compared to the control. Further, the number of heterophils and lymphocytes increased greater and greater in group treated with 50% and 60% of curcumin (Table 1).
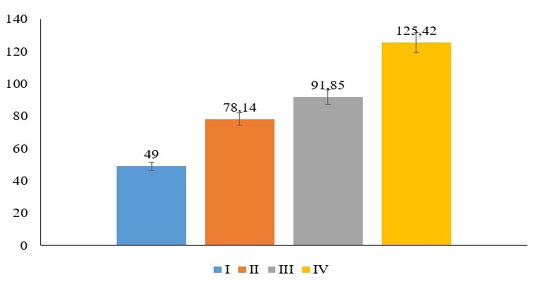
Further, the score of proliferation of white pulp from the chicken’s spleen showed significant increasing compared to the control group (p≤0.05). White pulp proliferation increased concomitantly along to the doses of curcumin. Group IV showed higher proliferation of white pulp and it followed by group III, II and I (Figure 1). Group I showed minimal proliferation of white pulp (Figure 2A and B). In contrast, there is an increasing regarding the lymphocytes proliferation within white pulp of spleen in group treated with curcumin (Figure 2C-H). It proved that curcumin derived from turmeric extract increase the proliferation of the lymphocytes within spleen tissue along to the increase of exposure doses (Figure 2).
Spleen is one of the potential organ on chicken in supporting and regulating the immune system. As the one of secondary lymphoid organ, spleen promote the proliferation of the lymphocytes and act as the site of antigen presenting cells (Sandford et al., 2011). The antigen presenting cells commonly occurs within the white pulp. White pulp is the immunologic region of the immune system within splenic
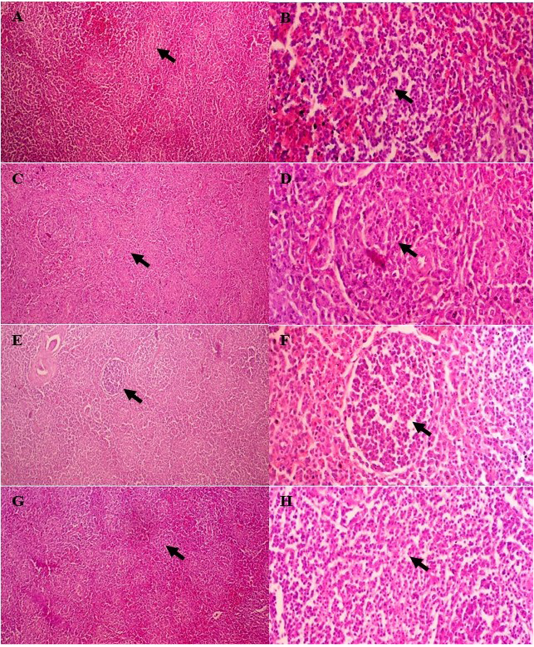
Figure 2: Spleen histopathology of broiler chicken treated with curcumin. White pulp of group I with minimal proliferation of lymphocytes (A and B); mild proliferation of lymphocytes within white pulp of group II (C and D); moderate proliferation in group III (E and F); high proliferation in group IV (G and H). H&E, A, C, E, F (100×); B, D, G, H (400×).
tissue. In this tissue, there is a lot of the white blood cells including lymphocytes (Zhang et al, 2017). The lymphocytes is the main actor that not only support in eliminating of the antigen but also synthesize the antibody in a normal condition, pathological condition (infection) (Sekelova et al., 2017), and or after exposure of the vaccine. Based on this mechanism, we know that lymphocytes play a significant role in the immune system. However, the proliferation of the splenic tissue and the lymphocytes inside it not only occurs in a simple pathway, but sometimes in intricate mechanism while involve the act of the antigen such as AI virus.
AI virus promote the high morbidity and mortality among the poultry industry. This virus increase within the blood stream and it impacts on the severe hemorrhage of the host body (Muramoto et al., 2006). The negative impact of AI virus infection can be prevented using either, vaccination and the utilization of immunostimulator such as curcumin derived from the turmeric extract. This study proved that curcumin increase the proliferation of lymphocytes within white pulp tissue. The high proliferation after the treatment using curcumin indicated that curcumin potentially increase the cellular immune responses of the host body. The increase of the lymphocytes not only occurs within the splenic tissue but also in the blood stream. The number of circulatory lymphocytes within the blood stream indicate that the mechanism of immune response become more attractive. The representation of circulatory lymphocytes in the blood after the treatment using curcumin show that the cellular response of the chicken body occurs in both tissue and circulatory system.
Another potential cells that play significant role in preventing the infection is heterophil. Heterophil is one of the granulocytes white blood cells in the first lineage of cellular response. All the pathogenic agent that enter to the body will be inhibited by the heterophl via the synthesized enzyme of its granule (Redmond et al., 2011). The curcumin promote the ability of heterophil to recognize the antigen, such as AI virus. It was proved by the utilization of curcumin in increasing circulatory heterophil in the chicken after vaccination. Greater number both of heterophil and lymphocytes in this study proved that curcumin act as the immunostimulator.
Further, the increase of the lymphocytes in group treated with curcumin was followed by the producing of antibody against AI after vaccination. It can be explained because the lymphocytes synthesize antibody and the higher activated lymphocytes commonly followed by the higher titer antibody. It is similar to the Abou-Elkhair et al. (2014) proved the potency of curcumin on promoting the greater titer antibody in broiler chickens. The mechanism of curcumin in increasing the lymphocytes and titer antibody against AI virus was suspected through the activation of the antigen presenting cells that influence on the B cells to regulate the presentation of antibody and T lymphocytes in direct killing of the antigen.
Conclusions
The administration of curcumin derived from turmeric extract potentially increases the immunity of broiler chickens via its ability to promote titer antibody, heterophil, lymphocytes, and proliferation of white pulp after vaccination. The 50% and 60% concentration of curcumin can be used as the alternative immunomodulator in poultry industry.
Author’s contribution
All the author contributed equally during the experiment, collecting data, analyzing data, and writing the manuscript.
Conflict of interest
The authors have no conflict of interest.
References