Advances in Animal and Veterinary Sciences
Research Article
Seroprevalence of Melioidosis in Sheep and Goats from Selected Small Ruminant Farms in Selangor, Malaysia
Idris Umar Hambali1,4, Yusuf Abba2,4, Asinamai Athliamai Bitrus2,6, Innocent Damudu Peter1,4, Faez Firdaus Abdullah Jesse1,3*, Thiviya Balakrishnan1, Mohd Azmi Mohd Lila2, Abd Wahid Haron1, Abdul Rahman Omar2, Nurizan Ahmad5, Fatiha Shuhaimy5
1Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia;2Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia;3Institute of Tropical Agriculture and Food security, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia;4Faculty of Veterinary Medicine, University of Maiduguri. P.M.B 1069 Maiduguri, Borno Nigeria;5Veterinary Research Institute (VRI), Department of Veterinary Services of Malaysia;6Research Unit, Microbial Food Safety and Antimicrobial resistance, Department of Veterinary Public Health, Faculty of Veterinary Science, Chulalongkorn University, 10330 Pathumwan Bangkok, Thailand.
Abstract | This study was designed to investigate the seroprevalence of Melioidosis in sheep and goats from selected small ruminant farms in Selangor, Malaysia. Blood samples (n=100) were collected each from sheep and goats using a random sampling technique from these farms. The serum samples were subjected to Melioidosis antibody screening using the Complement Fixation Test (CFT). The overall prevalence of Melioidosis among goats and sheep from these farms were 1% and 0%, respectively. It is concluded that although the prevalence of positive detection is low, there is need for further diagnostic surveillance as this singular case can be a potential reservoir for zoonotic infection.
Keywords | Burkholderia pseudomallei, Melioidosis, Complement Fixation Test (CFT), Seroprevalence, Sheep
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | December 05, 2017; Accepted | January 24, 2018; Published | February 15, 2018
*Correspondence | Faez Firdaus Abdullah Jesse, Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia; Email: jesse@upm.edu.my
Citation | Hambali IU, Abba Y, Bitrus AA, Peter ID, Jesse FFA, Balakrishnan T, Lila MAM, Haron AW, Omar AR, Ahmad N, Shuhaimy F (2018). Seroprevalence of melioidosis in sheep and goats from selected small ruminant farms in selangor, Malaysia. Adv. Anim. Vet. Sci. 6(2): 88-94.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.2.88.94
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Abba et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Melioidosis is an important neglected tropical disease caused by Burkholderia pseudomallei, a facultative anaerobic, non-spore forming, motile gram-negative bacillus found on both water and soil surfaces (Saif, 2013). It is found in pigs, cattle, humans, sheep, goats, horses, dogs and cats. This disease is of serious economic importance due to its effect on animal productivity, reduces valuable animal protein due to carcass condemnation (Brett and Woods, 2000) with a prevalence of about 13.6% in sheep and goats in Malaysia (Puthucheary, 2009) . Its zoonotic potentials poses a serious threat to human health hence it is considered a disease of public health importance (Pal, 2005). The route of transmission is through the oral, nasal routes and open wounds contact with contaminated soil or water. The clinical signs of melioidosis include; hyperthermia, anorexia , lymphadenopathy, lung abscesses and pneumonia (White, 2003; Adler et al., 2009).
The gold standard method for diagnosis of melioidosis is based on isolation and identification of the organism from lesions and discharges from blood, nasal swab, wound exudates, pus or tissues samples of infected of animals (Wiersinga et al., 2012). For immunological method, the most common approach is antibody detection as it is simple and requires minimal laboratory equipment. Effective serologic screening tests include complement fixation test (CFT) and indirect hemagglutination (IHA), Enzyme Link Immuno Serological Assay (ELISA) and immunofluorescence antibody test (IFA) (Sirisinha, 2000). PCR detection method also can be used in diagnosis of this disease (Chantratita et al., 2007). Serology technique has always been used for detection B. pseudomallei of anti- Burkholderia antibodies in horses, goats and dairy cattle in veterinary diagnosis (Thomas et al., 1988; Kingsley et al., 2016). CFT was preferred as screening test because of its high specificity in serodiagnosis of melioidosis (Thomas et al., 1988). The sensitivity of the CFT in for diagnosis of melioidosis in animals was estimated to be between 79.3 and 82.4% while its specificity was between 99.5 and 100%, which qualifies the test to be a good screening test (Thomas et al., 1988).
In Malaysia, the disease was first reported in animals in 1913 (Stanton, 1932). Since then, several cases of melioidosis in human and animals have been reported (Strauss et al., 1969; Aziz et al., 2005; Puthucheary, 2009; Deris et al., 2010; Musa et al., 2012). Recent available data on the occurrence of Melioidosis in Malaysia was provided by Naama et al. (2012), where sheep and goats had an incidence rate of 0.454% and 0.450% respectively. This study aimed to investigate the status of melioidosis and the risk factors associated with the seroprevalence of melioidosis among small ruminants from selected small ruminant farms in Selangor.
Materials and Methods
Study Area and Design
This study was designed to investigate the seroprevalence of melioiodosis among small ruminants (sheep and goat). The study involved selection of farms around Negri Sembilan (Sepang and Nilai) and Selangor (Serdang, Dengkil and Hulu langkat) area with no known prevalence of melioidosis. A total of 200 small ruminants were selected which comprises of Goats (n=100) and Sheep (n=100) based on convenient random sampling. Questionnaire was administered to identify risk factors associated with melioidosis. Factors considered include, management system, source of water, source of animals (Indigenous or imported) Biosecurity and history of disease.
Sample Collection
A total of 200 animals (sheep; n=100; goat; n=100) were randomly selected from farms around Selangor and Negri Sembilan. Each goat and sheep was physically restrained, and the site of jugular venipuncture was disinfected using 70% alcohol swab. Three milliliters (3mL) of blood was obtained from each animal via jugular venipuncture into pre-labelled vacutainer tubes (5mL each into plain anticoagulant free tubes). The samples were transported in an ice box to the laboratory for serological analysis.
Serum Extraction
Serum samples were harvested by centrifugation of blood at 3000 revolutions per minute (rpm) for 5 minutes (754 x g). The harvested serum were transferred into a fresh 1.5 mL microcentrifuge tube (Eppendorf). Serum samples were harvested in duplicates and stored at -20°C and -80°C respectively.
Laboratory Diagnosis of Burkholderia Pseudomallei
Complement fixation test (CFT) was used in screening and monitoring of the levels of antibodies against B. pseudomallei. The tests were performed at the reference laboratory for animal diseases in VRI, Department of Veterinary Services Malaysia, in parenthesis : (DVS)
Procedure of CFT
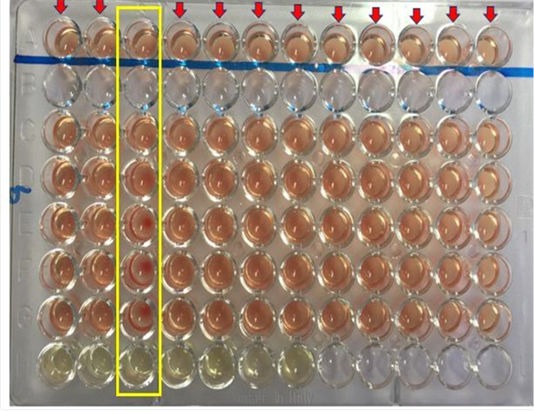
The serological test employed in this study to investigate the presence of antibodies against Burkholderia pseudomallei is the Complement Fixation Test (CFT). The test was performed as described according to the protocol recommended by the OIE (OIE, 2015). Briefly, the harvested serum sample were inactivated by heating in a water bath set at 63 °C for 50 minutes. A 64 well microtiter plate was used for the CFT. The wells were labelled from A to H, well A and H served as positive and negative control. Aliquot of 25 µL of the diluent was added into well A (control well to replace the melioidosis antigen), C, D, E, F, and G, while same amount (25 µL) of inactivated serum was transferred into well H, well B was left empty. The titration reaction was then performed from well H to C which include the addition of a standardized 25 µL of melioidosis antigen and melioidosis complement into each well. The plate was then incubated at 37°C for 15 minutes without shaking. After which 25 µL of the haemolytic system was added into each well A (control well), C, D, E, F, and G. The reaction was then thoroughly mixed using a shaker and then incubated at 37°C for another 15 minutes. After 15 minutes, the plates were shaken and left to settle at room temperature for 2-3 hours before reading the result. The reaction was considered positive for melioidosis if the antibody titre is 1: 8 or more.
ResultS
Cft Result Interpretation
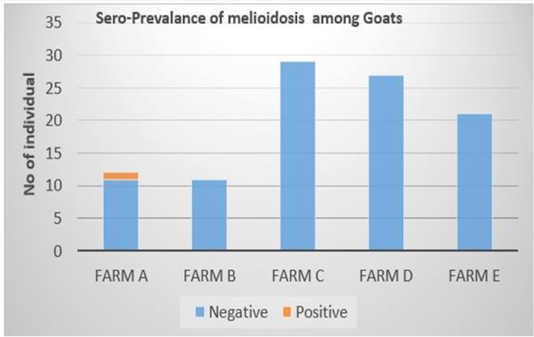
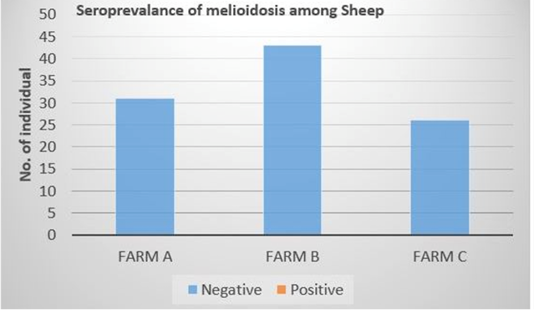
Out of the 100 serum samples collected from goat only one positive CFT reaction was observed in a complete row boxed in yellow colour (Figure 1) indicating no lysis hence pellet like formation suggesting presence of antibodies to B. pseudomallei. This was the only positive case observed as all other samples were negative with complete lysis observed indicative of the absence of antibodies to B. pseudomallei in those samples. This positive sample for B. pseudomallei was however traced back to animal samples obtained from Farm A (Figure 2) Similarly, none of the serum samples collected from sheep were positive for antibodies against B. pseudomallei. Thus, indicating the absence of antibodies to B. pseudomallei in those samples (Figure 3).
Questionnaire Data
The data generated based on the response of the respondents which served as description of the farms sampled showed that 80% (4/5) of the goat farms were located in Selangor while only 20% (1/5) was located in Negri Sembilan. Additionally, all the sheep farms sampled 100% (3/3)were also located in Selangor area. The data also showed that all farms were private owned; 40% (2/5) and 66.7% (2/3) of the goat and sheep farms practiced semi-intensive management system. While 60% (3/5) and 33.3% (1/3) of the goat and sheep farms practiced intensive management system. The result also showed that, all the farms sampled were housed in raised floor with slatted flooring. A total of 60% (3/5) of the sampled farms raised goats alongside sheep and 40% (2/5) do not rear goat alongside other animal species. While 100% (3/3) of the sampled sheep farms rear goat that are housed side by side.
For biosecurity of the farm, 80% of the goat farms in this study practiced importation of goats from other countries such as Australia and Indonesia while 20% does not practice importation. The animals were sourced from indigenous farmers raising local breeds of goat and sheep. For sheep farms, all animals were imported from Australia. For isolation or quarantine facilities in their farms only 80% goat farms has the facility whereas all sheep farms in this study provides isolation or quarantine facilities. The entire enrolled farm in this study practises daily cleaning of the farm without the use of any disinfectant. The animals in these farms are provided with cut and carry grass and treated water. The farms do not perform any disease monitoring program or screening and keeps good herd records.
Discussion
Melioidosis is an infectious disease of great concern to public health inspectors; it affects terrestrial and aquatic animals in Southeast Asia and other parts of the Tropics (Musa et al., 2012). There is increase dissemination of the organism worldwide this could probably be due to increase in the rate of global migration of people and animals thus, increasing the overall risk of the disease going beyond areas or regions considered to be endemic with melioidosis (Musa et al., 2015). As part of Flood Action Plan that precedes the 2006-2007 flood disaster in Johor, the disease is included in the list of notifiable communicable disease in humans in Malaysia (Hisham et al., 2009; Musa et al., 2015). Additionally, shortly after the 2004 tsunami that causes widespread devastation in some parts of Asia, high incidence of melioidosis was also reported (Athan et al., 2005; Chierakul et al., 2005; Wuthiekanun et al., 2006). The increase in the number of human cases reported in the studies indicated an extensive contact between animals and flood water contaminated with B. pseudomallei.
This present study investigated the seroprevalence of melioidosis in sheep and goats from randomly selected small ruminant farms in Selangor using CFT technique. The overall seroprevalence of meliodosis among goats and sheep in the farms sampled were 1% and 0% respectively. Malaysia is known to be one of the endemic countries for melioidosis disease outbreak worldwide as the organism is a natural ubiquitous soil-dwelling saprophyte bacterium (Cheng et al., 2005). Therefore, the 1% positive detection of seroprevalance in this study may due to the endemicity of this disease in Malaysia where the climate in Malaysia is very favorable for the survival of the organism (Cheng et al., 2005). Moreover, the seropositive detected animal in this study is from the farm that practice semi-intensive farm management system whereby the animals are allowed to graze freely in the morning and evening. This may expose the seropositive detected animal to moist clay soil and access to mud that seem to be favored by the organism (Musa et al., 2016). Several studies have reported the likelihood of increased isolation rate of B. pseudomallei in soil with high clay content (Dance, 2000; Musa et al., 2016). This is because the high nutrient and water retaining capabilities of clay soil coupled with it large surface area and chemical activity supports the survivability of B. pseudomallei (Na-ngam et al., 2004; Kaestli et al., 2009; Sommanustweechai et al., 2013). Thus, indicating that goats raised semi-intensively have high chance of coming down with melioidosis. The water source supplied to the animals sampled in this study are all wholesome water which has already been treated in water treatment plant. Thus, it reduces possibility of spread of disease compared to groundwater source or stagnant water. Therefore, this reduces the possibility that water supply is the source of infection for the seropositive detected animal in this study.
The result of the present study is however lower than the previous incidence of Melioidosis reported by Naama et al. (2012) and Kasantikul et al. (2015). The differences may be due to difference endemicity of the disease the area studied, difference in breeds of the animals, this is because susceptibility to the disease varies across different breeds. In addition, difference could be due to the difference in the number of farms sampled and the number of animals sampled. This study sampled only 8 farms and in all the farms only 200 animals were selected and sampled, this could also affect the seroprevalence of the disease. The management practice can also affect the incidence of the disease; since, it is expected that farms that intensively raised their animals should have low or no seroprevalence of melioidosis as opposed to those farms that practice semi-intensive management system. Regular screening of seroprevalence of melioidosis is crucial as reported by Abdullah et al. (2015), the authors recommend compliance to screening of animals and disease monitoring programs. Although, the seroprevalence rate is low in this study, emphasis should be given due to the serious public health importance of melioidosis since it is a zoonotic infection causing serious economic problems (Choy et al., 2000). In addition, the fact that the disease is present in farm A also poses a significant public health challenge to the farmers and people living around the farm. This is because, melioidosis is endemic in Malaysia and its transmission is via inhalation or subcutaneous inhalation of contaminated soil and water. Furthermore, since the incidence of the disease is high during raining season also suggest the need for a thorough screening of all farms, soil, water and environmental surfaces around the farm area (Ulett et al., 2001; Currie and Jacups, 2003). In a retrospective study conducted in Thailand from 2006 to 2010, Limmathurotsakul et al. (2012) reported high incidence of melioidosis in humans (1.63/100,000/ year), this was followed by incidence in pigs (0.02/ 100,000/year) and cattle (0.01/100,000/year) respectively. The authors also showed that the incidence of melioidosis in goats in a given region paralleled that of humans. This further confirmed the endemicity of the disease in Southeast Asia and the impending public health and economic consequence associated with the incidence of the disease. The presence of the disease in farm A can also be associated with management practice of the farm as well as the source of the animals. Since animals from farm A were sourced from Australia and Indonesia also suggest that the animals most have been exposed before they were imported into the country. Even though the true prevalence of melioidosis in Malaysia, is still unknown, an overall seroprevalence of 5.7% among livestock has been reported. In addition, 2.6% and 13.6% reactor rates have been reported by Musa et al. (2012). Furthermore, a 14% prevalence of melioidosis have been reported in Australia (Currie et al., 2004; Limmathurotsakul et al., 2013).
Conclusion
This study provides data on seroprevalence of melioidosis in small ruminants from selected farms in Selangor, Malaysia using complement fixation test (CFT) technique. There is need for further diagnostic surveillance as seropositive detection was made in this study and this can be a potential reservoir of the spread of the disease.
Conflict of Interest
There is no conflicting interest with regards to the publication of this research work.
ACKNOWLEDGEMENTS
The authors wish to appreciate the management and staff of Universiti Veterinary Hospital (UVH), Universiti Putra Malaysia (UPM) and Veterinary Research Institute (VRI), Department of Veterinary Services (DVS) Malaysia for their cooperation throughout the course of this study.
Authors Contribution
All authors contributed equally.
References
Supplementary Material
Appendix A: Questionnaire related to management, biosecurity and medical history for each sampled farm
|
Farm's background |
|||
|
Farm's Owner: |
|||
|
Year of establishment: |
|||
|
Type of farm (please tick):
|
[ ] Government
|
[ ] Small holder |
[ ] Others:
|
|
Animal purposes (please tick): |
[ ] Meat
|
[ ] Dairy
|
[ ] Others:
|
|
Breed of Goats: |
|
|
|
|
Farm's population: |
Total: |
|
|
|
|
Male: |
|
|
|
|
Female: |
|
|
|
|
Young: |
|
|
|
Animal Performance in Farm |
|||
|
General performance: |
[ ] Fair
|
[ ] Good
|
[ ] Excellent
|
|
Housing Management |
|||
|
Type of housing: |
[ ] Raised |
|
[ ] Others: |
|
Management system: |
[ ] Intensive |
|
|
|
|
[ ] Semi-intensive |
|
|
|
|
[ ] Extensive |
|
|
|
Farm Biosecurity and Disease Management and Control |
|||
|
Any nearby farm: |
[ ] Yes |
|
|
|
|
[ ] No |
|
|
|
Importation of Animals: |
[ ] Yes |
Country of origin: |
|
|
|
[ ] No |
|
|
|
Quarantine practice: |
[ ] Yes |
Where: |
|
|
|
[ ]No |
|
|
|
Water source: |
[ ] Treated |
|
|
|
|
[ ]Groundwater |
|
|
|
|
[ ]Stagnant water |
|
|
|
Bush Cleaning: |
[ ] Yes |
|
[ ] No |
|
Soil liming: |
[ ] Yes |
|
[ ] No |
|
Previous disease outbreak: |
|
|
|