Advances in Animal and Veterinary Sciences
Research Article
Anticancer and Antioxidant Activity by Secondary Metabolites of Aspergillus fumigatus
Taghreed N. Almanaa1*, Marwa A. Yassin2, Rasha M. El-Mekkawy2, Noha Saleh Ahmed2*, Gamal Hassan Rabie2
1Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia; 2Department of Botany and Microbiology, Faculty of Science, Zagazig University, Zagazig, Egypt.
Abstract | Due to the challenge faced scientists to produce the natural product as an anticancer agent from cheap sources and in endeavor to ameliorate the rate of anticancer agents, the employment of fungal metabolite in creating a cordial proper process was prerequisite. In the present study, fungal metabolites are detected by thin layer chromatography. The fungal producer strain was isolated from the rhizosphere region of old cultivated soil. One fungal isolate out of bioassay ten isolates was showed to have the most potent anticancer and antioxidant activity; this fungal isolate was identified as belonging to Aspergillus fumigatus (A. fumigatus). Fungal extract of A. fumigatus showed an antioxidant activity using Diphenyl-1-picrylhydrazyl (DPPH) scavenging % at 94.5±0.70 with IC50 at 5µg/ mL. To elucidate chemical analysis of the different bioactive compounds; A. Fumigatus metabolites extract was subjected to instrumental analysis such as GC. Mass. The A. fumigatus metabolite showed a promising anticancer activity. Inhibitory activity against Hepatocellular carcinoma cells was detected under these experimental conditions with IC50= 113 ± 3.7 µg/ml. This property can be further used to formulate new age drugs.
Keywords | Aspergillus fumigatus, Antioxidant activity, GC-Mass, Anticancer activity
Received | September 26, 2020; Accepted | November 03, 2020; Published | January 01, 2021
*Correspondence | Taghreed N. Almanaa and Noha Saleh, Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia; Department of Botany and Microbiology, Faculty of Science, Zagazig University, Zagazig, Egypt; Email: talmanaa@ksu.edu.sa; Ns7_noon@yahoo.com
Citation | Almanaa TN, Yassin MA, El-Mekkawy RM, Ahmed NS, Rabie GH (2021). Anticancer and antioxidant activity by secondary metabolites of Aspergillus fumigatus. Adv. Anim. Vet. Sci. 9(2): 265-273.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.2.265.273
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Almanaa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cancer and Reactive oxygen species (ROSs) have turn into numerous issues of disease also death (Smyth et al., 1998; Shi et al., 2007). The biocontrol of these ROSs and cancer are of interest to persist study to detect and discover newly solution to prevent such disease in vitro. In this regard, there is an inevitable and urgent medical need for natural bioactive metabolites with novel anticancer and antioxidant activity (Enan et al., 2018; Abdelshafi et al., 2020; El-Sayed et al., 2020) and there is frequently concern in detection protocols to create confident and appreciate-efficient biocidal products (El-Gazzar and Ismail, 2020; El-Gazzar et al., 2020; Enan et al., 2020).
Fungi are the most diverse for a broad assortment of secondary derivative products, that distinct primary extracts, perform a potent function in the biological operation of organisms (Ronsberg et al., 20l3; El-Gazzar and Enan, 2020). Recently, investigation procedures subjected for diverse fungi that have been confirmed their roles in curative fields (Simmon et al., 2005). Derivative extracts output from fungal strains are characterized by possess incoming innate outputs which subjected in medicine and industries features (Suryanarayanan et al., 2009; Ronsberg et al., 2013). Whereas, thin layer chromatography was employed successfully for the separation of different bioactive compounds (Dedio et al., 1969).
Aspergillus sp. are ubiquitous opportunistic moulds that are ethologically and therapeutically important (Yan et al., 2008). Many literatures reported numerous bioactive metabolites isolated from Aspergillus sp. (Huang et al., 2009; Nadanaciva and Will, 2009). These metabolites showed significance therapeutic importance such as anticancer and antioxidant activities. The biological value of this fungal species, make it of considerable interest to the scientific. There are necessities to persist study to detect compounds with effective anticancer activity. In consideration, this work endeavors to detect the role of bioactive metabolites by Aspergillus fumigatus as anticancer and antioxidant agent.
MATERIALS AND METHODS
Chemicals
Czapek Dox media (NaNO3–Sucrose-Kcl-MgSO4–KH2PO4), agar, PDA media (glucose yeast extract potato filtrate), chloroform, methylene chloride, ethyl acetate, methanol, acetonitrile, dimethyl sulfoxide (DMSO), hexan, toluene, ethanol 95%, sodium carbonate (7.5% w/v), sodium nitrite (5% NaNO2, w/v), sodium hydroxide (4% NaOH, w/v), 2,2-Diphenyl-1-picrylhydrazyl (DPPH).
Fungal strains: Isolation and correspondence
The fungal strains used in this study were isolated from rhizosphere region of soil contaminated with wastes from Photographic Industries (El-Sharkia Governorate, 80 km North Cairo, Egypt) (El-Gazzar, 2015). They were purified and finally grown in slants of Czapeks Dox medium. The isolates were identified as reported in Raper and Thom (1949), Raper and Fennell (1965), Booth (1971).
Preparation of fungal inocula
The fungal cultures used in this investigation were subjected for spore production through the growing of the tested fungi on PDA medium at about one week at 28±2°C. The spore suspension was purified by cheesecloth; then nominate. Spores were enumerated by qualified hemocytometer. A convenient attenuation were prepared using the supply spore narrator through sterilized 0.1% (w/v) peptone water as diluents to take out the in demand spores level of 4x102 cells/ mL (Ellis et al., 1991).
Fungal metabolite extraction
Metabolites were extracted from the mycelium that inoculated in an autoclaved Czapek Dox Broth (100 ml) at121°C, 15 lbs pressure for 15-20 min in Erlenmeyer flask and incubated in dark (10 d, 25 ± 1°C).
Extraction of intracellular secondary metabolites
The fresh mycelial mat of each fungal strain was ground in a mortar in the presence of sterile fine sand and methanol: chloroform (1:2 v/v) to extract the secondary metabolites then centrifuged and filtered. The solvent was evaporated, using an air drier and the metabolites were dissolved in methanol and stored at 5 °C.
Extraction of extracellular secondary metabolites
The broth filtrates were defatted with n-hexane, then collected and taken away using methanol: Chloroform (1:2 v/v) in a separating funnel, shaken well and left at least for six hours until complete separation from the lower phase. Sediment was re-taken away again for full separation by evaporation of solvents and the metabolites were dissolved in methanol and stored at 5°C.
The detection of fungal secondary metabolites by using TLC
The secondary metabolites such can be employed. After incubation period, the fungal mats were separated from the culture by filtration then metabolized medium was collected. The broth filtrates can be applied on thin layer chromatography TLC plates. Developing processes can be carried out, using chloroform: methanol (9: 1 v/v) system for separation of fungal secondary metabolites (Dedio et al., 1969).
Antioxidant efficiency of fungal secondary metabolites
The antioxidant efficiency of the extracts dissolved in methanol was determined with the standard of their scavenging efficiency of the 1, 1- diphenyl-2-picryl hydrazyl (DPPH) free radical Melo et al. (2008). The alleviation of DPPH by antioxidant extracts or metabolites creates a forfeit of absorbance. So, the stage of discolouration of the sol shows the scavenging effectiveness of the applied matter. While DPPH reacts with an antioxidant constituent that can confer hydrogen atoms, it decreases and changes intensity of dark violet to sprightly-yellow. Trail pattern were set through fluxing in dimethyl sulfoxide (DMSO), with mixtures of 10 L of trail specimen and 90 L of PPH (final concentration of test sample was 500 g/ mL and 300 a mol of DPPH was added then put at 37°C for 30 minutes, Absorbance was examined at 517 nm with spectrophotometer. Ascorbic acid was used as a positive standard. The study was performed in triplicate. Scavenging efficiency rate was determined using the subsequent formula: Scavenging activity (%) = [(A control – A sample)/A control] × 100.
Antitumor activity of the most potent fungal secondary metabolites
Subculture of cell lines
The cell lines were maintained in RPMI 1640 (Life Technologies, Inc., Grand Island, NY) supplemented with 50 μg/ml gentamycin and 10% (v/v) heat inoperative fetal bovine serum (FBS) and developed at 37°C in 5% CO2 (Water Jacketed Double door incubator, Shel Lab, Sheldon Manufacturing, Inc.®, USA) about 48 hrs. Samples were examined with an inverted microscope (CKX41; Olympus, Japan) to check the cultures and to confirm their sterility from contamination by microorganisms. Cells layer rinsed using phosphate buffered saline, pH 7.2 without Ca2+/Mg2 +, a volume equal to semi the cultivated medium size. Trypsin/ EDTA was introduced into the rinsed single layer cell using 1 mL per 25 cm2 of surface area and left in 37°C about 5 minutes. Then, the samples were examined by a microscope to confirm their detached and floated. They were combined with new FBS containing RPMI environment. The count of samples was measured by taking away 100-200 μL from their followed by trypan blue dye exclusion method using a hemocytometer. The in demanded count of samples was put in plates supplied with growth environment then kept as recommended for the cell line.
Estimation of the anticancer efficiency
The antitumor activity was evaluated on single carcinoma cell lines, namely HepG2 cell. The cell line was originated as mono layers with 10 per cent (v / v) inoperative foetal calf serum and 50 μg / mL gentamycin in the growth medium. The single layers of 10,000 organisms subjected at down of the 96-bore plate kept at 37ºC about 24 h and 5% CO2. The cells were then rinsed by phosphate buffered saline (0.01 M; pH 7.2) then they were subjected by 100 µL of diverse concentration of fungal extracts or pure metabolites new environment and kept at 37ºC. The control of unhandled cells was applied without strains products. Then, the count of handled cells was determined after one day of incubation by staining these cells with crystal violet (0.1 %, w/v) subsequence with rapture using 33% glacial acetic acid and examining at 590 nm using ELISA (Model: Sunrise, Tecan Inc., USA) after good combination. The readings of controlled cells were pointed as 100% proliferation (Mosmann, 1983; Vijayan et al., 2004). The rate of cell efficiency was measured by [1-(ODt/ODc)] x100%; whereas, ODt is the medium optical density of the strain product handled wells and ODc is the optical density medium of the control sample
Gas chromatography of the most active fungal secondary metabolites
Secondary products of the potent effective fungal strain were subjected by GC/mass procedure: Gas Thermo chromatography 1310 connected with ISQ LT single quadrupole mass spectrometer at department of fungi, Al-Azhar University, Egypt. The procedure was integrate canicular line (DB1 JandW; 30 m length; 0.25mm Inner diameter; 1.5 um film thickness), that synthetically fixed dimethyl polysiloxane. The procedure was adjusted at 40°C (1 min) then elevated to 250°C (2 min) at a rate of 5°C/min and then elevated to 310°C (2 min) at a rate of 5°C/min. The temperature for the detector and the injector was set at 300° C. WILEY information spectrum basis was employed for the discrete peaks consistency.
RESULTS
Isolation of fungi and a preliminary identification of isolates
In the present investigation, ten fungal isolates which isolated from the rhizosphere of old cultivated soil were summarized in Table 1. The obtained data in Table 1 emphasized that preliminary identification of the ten fungal isolates, by international Reference Key. Under investigation, these isolates were affiliated to namely Aspergillus fumigatus, A. terrus, A. flavus, A. oryza, Alternaria alternata, Rhizopus sp., Penicillium citrinum, Fusarium oxysporium, Cunninghamella sp. and Trichoderma sp.
Table 1: Number of secondary metabolites separated on TLC and its antioxidant activities of conc (50 µg/mL).
|
DPPH scavenging % |
Fungal isolates | No. | |
| Extra. | Intra. | ||
| 94.5±0.70 | 76.3±1.45 | Aspergillus fumigatus | 1 |
| 51.7±1.22 | 23.1±0.55 | Aspergillus flavus | 2 |
| 74.2±1.19 | - | Aspergillus terrus | 3 |
| 22.9±1.31 | - | Rhizopus sp. | 4 |
| 17.6±0.52 | - | Alternaria alternata | 5 |
| 12.3± 0.55 | - | Fusarium oxysporium | 6 |
| 21.6±0.16 | 17.7±0.51 | Penicillium citrinum. | 7 |
| 46.3±0.85 | 19.6±0.66 | Cunninghamella sp. | 8 |
| 77.4±0.65 | 56.6±0.65 |
Trichoderma sp. |
9 |
| 47.5±0.45 | 21.5±0.60 | Aspergillius oryza | 10 |
Biological activity of secondary metabolites from fungal isolates
In this study, 100 ml of each culture broth of ten tested filamentous fungi were taken away using (2:1 v/v) in a separating funnel. Each fungal extract was concentrated, then collected and stored for further studies. Each fungal extract was applied on TLC silica gel plate and optimized by developing solvent system (chloroform and methanol) in the ratio (9:1 v/v) for separation of the secondary metabolites into individual metabolites. From Table 1 and Figure 1, it was shown that fungal secondary metabolites constitute a huge array of low molecular weight natural products with a wide range of chemical diversity.
A significant antioxidant activity was noted in most cases ass shown in Table 1 and Figure 1. However, weak antioxidant action was examined in the intracellular extracts also extracellular outputs of fungal isolates A.terrus, Rhizopus sp., A. alternata and F. oxysporium. On the other hand, marked antioxidant action was investigated in the intracellular extracts also extracellular outputs of fungal isolates Pencillium citrinium and cunnininghamella sp. In addition, strong antioxidant activity was detected in the intracellular extracts and extracellular extracts of fungal isolates A. fumigatus, A. flavus, Trichoderma sp. and A. terrus.
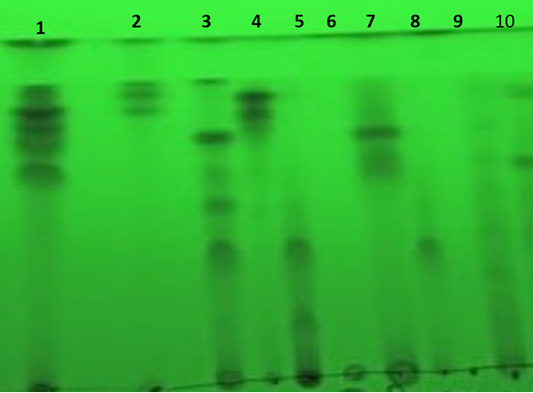
Figure 1: Separation of extracellular secondary metabolites on TLC plate under short wavelength UV light. Where, the numbers from 1 to 10 are the numbers of fungi in the Table 1.
Antioxidant activities of the most potent extracellular secondary metabolites
Antioxidant activity of the most potent A. fumigatus extract was evaluated by using DPPH scavenging. The A. fumigatus extract showed an antioxidant activity under these experimental conditions with IC50 = 5 µg/ml as shown in Table 2. In addition, the sample of Ascorbic acid reference standard showed an antioxidant activity under these experimental conditions with IC50=5 µg/ mL as shown in the Table 3.
Table 2: Antioxidant activity of extract of Aspergillus fumigatus.
| Sample conc. (µg/mL) | DPPH scavenging % |
| 640 | 82.44 |
| 320 | 79.33 |
| 160 | 67.11 |
| 80 | 48.22 |
| 40 | 41.56 |
| 20 | 21.33 |
| 10 | 13.33 |
| 5 | 9.78 |
| 0 | 0 |
Antitumor activity of the most potent extracellular secondary metabolites on HCT and H (liver carcinoma cell line)
Inhibitory activity of metabolites extract of A. fumigatus against hepatocellular carcinoma cells was detected under these experimental conditions with IC50= 113 ± 3.7 µg/ml. as shown in Table 4 and Figure 2. Gradual decreasing of tumor cells when the concentration of fungal extract increased as shown in Figure 3.
Table 3: Antioxidant activity of ascorbic acid reference standard.
| Sample conc. (µg/mL) | DPPH scavenging % |
| 40 | 92.48 |
| 35 | 87.53 |
| 30 | 80.65 |
| 25 | 77.41 |
| 20 | 70.94 |
| 15 | 54.86 |
| 10 | 17.49 |
| 5 | 11.78 |
| 0 | 0 |
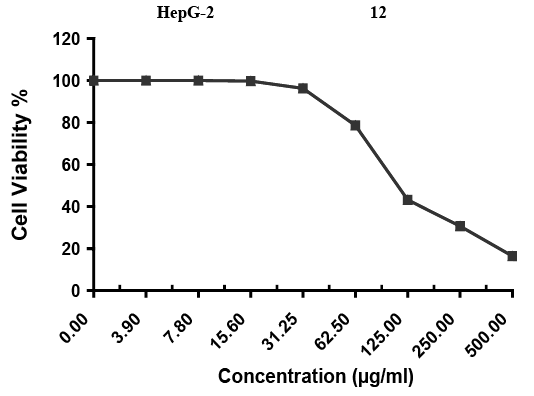
Figure 2: Evaluation of cytotoxicity against HepG-2 cell line; Inhibitory activity against Hepatocellular carcinoma cells was detected under these experimental conditions with IC50 = 113 ± 3.7 µg/ml.
Table 4: Evaluation of cytotoxicity of A. fumigates extract against HepG-2 cell line.
| Sample conc. (µg/ml) | Viability % | Inhibitory % | S.D. (±) |
| 500 | 16.39 | 83.61 | 2.14 |
| 250 | 30.62 | 69.38 | 1.36 |
| 125 | 43.18 | 56.82 | 3.46 |
| 62.5 | 78.61 | 21.39 | 2.75 |
| 31.25 | 96.24 | 3.76 | 0.98 |
| 15.6 | 99.73 | 0.27 | 0.25 |
| 7.8 | 100 | 0 | |
| 3.9 | 100 | 0 | |
| 0 | 100 | 0 |
Table 5: Chemical composition of 22 components from A.fumigatus extract when subjected to GC-MS (gas liquid chromoatographic mass spectrometry).
| No. | Rt | M.formula | M.W. | Compound name and structure | Area |
Parent ion (M+) |
Base Peak (m/e) (100%) | Biological activity |
| 1 | 8.77 |
C8H10O |
122 | Phenylethyl Alcohol | 1.16 | 122.0 | 92.00 |
Anticancerand Antioxidant (Kim et al., 2015) |
| 2 | 16.92 |
C12H16O5 |
240.0 | 3-FURANACETIC ACID,4_HEXYL_2,5_DIHYDRO_@<%_DIOOXO_ | 29.05 | 240.0 | 126.0 |
Anticancer (Jelena et al., 2015) |
| 3 | 17.23 |
C14H22O |
206 | Phenol, 2,4-Bis(1,1-DIMETHYLETHYL)_ | 4.66 | 206.0 | 161.0 |
Anticancerand Antioxidant (Kim et al., 2015) |
| 4 | 17.74 |
C12H17CLOSI |
240.0 | [(7-Chloro-2,3-dihydro-1H-inden-4-yl)oxy](trimethyl)Silane | 0.15 | 240 | 225.0 |
Antioxidant activity (Yadav et al., 2018) |
| 5 | 18.73 |
C16H32 |
224.0 | 1-HEXADECENE | 0.35 | 224.0 | 43.0 |
Anticancerand Antioxidant (Yan et al., 2013) |
| 6 | 19.37 |
C13H14N2O2 |
230.0 | 4H-BENZO[DE][1.6] NAHTHYRIDINE,5,6-DIHYDRO-8,9_DIMETHOXY_ | 0.63 | 230.0 | 215.0 |
Anticancer (Abeer et al., 2019) |
| 7 | 20.52 |
C14 H21NO3 |
251.0 | Phenol,2,4-di-t-butyl-6-nitro-CIS-4B,5 | 0.76 | 251.0 | 236.0 |
Anticancerand Antioxidant (Govinoappa et al., 2017) |
| 8 | 21.24 |
C14H22O2 |
222.0 | 1,4-Benzenediol,2,6-bis(1,1-dimethylethyl)- | 0.25 | 222.0 | 207.0 |
Antioxidant (Tamil et al., 2017) |
| 9 | 23.20 |
C23H38O2 |
346.0
|
6,9,12,15-Docosatetraenoic acid, methyl ester | 0.22 | 346.0 | 43.0 |
Antioxidant (Rajani et al., 2015) |
| 10 | 23.46 |
C14H24O3 |
240.0 | 12-Hydroxy 14 methyl-oxa- cyclotetradec-6-en-2-one | 0.36 | 240.0 | 67.0 |
Anticancer (Jelena, 2015) |
| 11 | 27.22 |
C14H13CL3O3 |
334.0 | 12-Oxotricyclo[5.3.1.1(2,6)]dodeca-3,8-diene,11-acetoxy-4,5,9-trichloro | 1.21 | 334.0 | 43.0 |
Antioxidant (Rajani et al., 2015) |
| 12 | 28.83 |
C11H9C12NO3 |
273.0 | 2,4-OXAZOLIDINEDIONE,3-(3,5-DICHLOROPHENYL0-5,5-DIMETHYL- | 0.64 | 273.0 | 43.0 and 186.0 |
Anticancer and Antioxidant (Karakus et al., 2018) |
| 13 | 28.94 |
C16H14F5NO4Si |
407.0 | (4-Methoxy-3-nitrophenyl) methano1,dimethylpentafluorophenylsilyl ether | 0.29 | 407.0 | 166.0 |
Anticancer and Antioxidant (Risa et al., 2019) |
| 14 | 28.94 |
C15H10C12N2O2 |
320.0 | 2H-1,4-BENZODIAZEPIN-2-ONE,7-Chloro-5-(2-Chlorophenyl)-1,3-DIHYDRO-3-HYDROXY- | 0.29 | 320.0 | 293.0 |
Anticancer and Antioxidant (Deepak et al., 2019) |
| 15 | 28.94 |
C15H10CL2O |
276.0 | (2E)-3-(3-Chlorophenyl)-1-(4-Chlorophenyl)-2-Propen-1-ONE | 0.29 | 276.0 | 139.0 |
Anticancer and Antioxidant (Risa et al., 2019) |
| 16 | 29.05 |
C25H23C1N2O2 |
418.0 | 1-(3-CHLOR-PHENYL)5-(2-METHOXY-PHENYL)-VINYL]-4,5-DIHYDRO-1H-PYRAZOLE | 0.32 | 418.0 | 91.0 |
Anticancer and Antioxidant (Karakus et al., 2018) |
| 17 | 34.66 |
C15H16O6 |
292.0 | Picrotoxinin | 0.31 | 292.0 | 43.0 |
Antioxidant (Lemoreaux, 2017) |
GC analysis of extracellular secondary metabolites for selected isolates
The GC analysis of extracts of A. fumigatus culture filtrate gave us seventeen major compounds with potent antioxidant and anticancer activities as shown in Figure 4 and Table 5.

Figure 4: GC-Mass of A. fumigatus extract.
DISCUSSION
Natural sources offer recently a promising interest to be used as therapy for either treatment of cancer or inhibition of multidrug pathogenic bacteria (Uzma, 2018; Abdel-Shafi et al., 2019, 2020; Enan et al., 2020). They possess an attractive prospective as they are safe agents. In the present study, the filamentous fungi were suggested to have a potential efficacy of some secondary metabolites as antioxidant and antitumor; this result agrees with the observations of Chen et al. (2011). In addition, the present investigation revealed that, the most prevalent fungi were Aspergillus fumigatus, A. flavus, A. oryza, Alterutaria alternata, Penicillium citrinium, Rhizopus sp. and Trichoderma; this result agrees with the observations of Tenguria and Khan (2011).
In the present investigation, the characterization of the secondary products of A. fumigatus showed that the presence of bioactive compounds that produced by other similar fungal organisms similarly to observations of Frisvad et al. (2005).
From the obtained results, all metabolites of diverse fungi showed an antioxidant activity up to varying extent. This result agrees with the observations of Kumaresan et al. (2015). All isolated fungi their team studied had antioxidant efficiency in vitro. In addition, estimation of bioactive outputs from A. fumigatus confirmed the existence of diverse natural drugs similar to that reported previously (Abubakar and Ndana, 2016). Moreover, the results reported that the presence of free radical scavenging power of antioxidant components. Parallel work related to antioxidation activity was done by Tejesvi et al. (2008); Ghasemzadeh et al. (2010).
The present study suggests that naturalistic potent products extracted from fungi act as sequential provenance for the detection of novel antiproliferative agents and this concurs with the previous reports (Alvin et al., 2014; Jalgaonwala et al., 2017).
From this investigation, the antitumor test of the selected fungal isolates showed that most of the fungal secondary metabolites (extracts) have antitumor activity due to the presence of bioactive natural compounds. This result agrees with the observations of Strobel (2003) who reported the production of diverse forms of bio-effective secondary products combined with most potent sides of flavonoids, phenols, alkaloids, terpenoids and others. Moreover, those effective products possess broad efficiency in medical fields. Thus, the presented results confirmed an exclusive activity of A. fumigatus extract against the HepG2 hepatocellular carcinoma cell line as reported previously (Victor et al., 20l8).
CONCLUSION
The efficient role of fungi bioactive products have been confirmed as strong in vitro cytotoxicity activities. Performing the GC-MS analysis of A. fumigatus extract, with potent phytochemicals confirmed its ability to induce the cytotoxic mechanism. The results indicated that the A. fumigatus had a potent naturalistic constituent in cytotoxicity. Additional researches are in demand for purification the bioactive products which can be discussed as efficient antiproliferative drugs.
ACKNOWLEGEMENT
The authors thank Prof. Dr. Gamal Enan, Dean of Faculty of science, Zagazig University, Egypt for his critical revising the manuscript.
AUTHOR’S CONTRIBUTION
All authors have made a substantial and intellectual contribution to the work, and confirmed it for publication.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFRENCES