Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (4): 218 – 221Aspergillus Fumigatus Infection among Dromedary Camels in Saudi Arabia
Fahad Al Hizab1*,
- Department of pathology, College of Veterinary Medicine and Animal Resources, King Faisal University, Saudi Arabia
*Corresponding author: mhamouda@kfu.edu.sa
ARTICLE CITATION:
Al Hizab F (2014). Comparative efficacy of limited contact–dynamic compression plate and dynamic compression plate for repair of diaphyseal femoral fracture in dogs. Adv. Anim. Vet. Sci. 2 (4): 218 – 221.
Received: 2014–01–31, Revised: 2014–03–15, Accepted: 2014–03–16
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.4.218.221
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
This study was conducted in Eastern Province (2012). The field problem was the mortality among camels (Camelus dromedarius) in Hardd region in King Saudi Arabia (KSA).Clinical signs were anorexia, depression, nasal discharges, serous lacrimation and enlargement of submandibular lymph nodes. Moreover, there was bloody diarrhea.Total of (n = 150) camels, out of which (n = 70) animals showed the clinical disease and among (n = 19) of them were dying within three days after the appearance of the first clinical sign. Consistent necropsy findings were hemorrhagic abomasal folds, massive hemorrhage within the small intestine associated with severely congested mesenteric lymph nodes. Epicardial and subendocardial hemorrhages were also observed. The pharyngeal and laryngeal areas were congested and contained serosanguinous fluid. Aspergillus fumigatus was cultured and isolated from trachea, lung, omasum and intestine. No other potential microorganisms or toxic agents have been identified.
INTRODUCTION
A. fumigatus, is associated with respiratory system infections in livestock, but may also cause gastroenteritis. Moldy litters are often suspected as sources of infection.A. fumigatus is an opportunistic fungus and has been recorded in alpacas and dromedaries (Bhatia et al., 1983; Pickett et al., 1985; Severo et al., 1989; Gareis and Wernery, 1994).
The majority of aspergillosis is sapronoses, that is transmissible from the environment, and not from person to person, or zoonosis (Alesandro, 2010). Genus Aspergillus is a wide spread fungi around the world. Among its types; A. fumigatus is the most pathogenic type causing diseases in human & animals. A. fumigatus is an opportunistic fungus affecting the respiratory system (Kwon–Chung, 1992). Elevated pathogenic manifestations of A. fumigatus belong to several factors; it's ability to grow faster than other types in a wide range of temperature (20–50) ºC, A. fumigatus is highly sporelating fungus for which it has found in about (1–100) spore/m³ in the atmosphere (Lacey, 1996).
A. fumigutus is responsible for 90–95% of Aspergillus infections in animals. Aspergillus species can produce colonies of different colors (black, green or yellow). These colors are due to pigmented spores. Aspergillus species may be invasive, cause mycotoxicoses and allergic reactions in humans (Taylor et al., 2009). A hemorrhagic disease in camels characterized by a variety of clinical signs and hemorrhages at a necropsy has been noticed by El–Khouly et al. (1992). Eastern Province is considered a good place for the growth of fungi because its climatic condition (Khalid AL–Busadah, 2007).
MATERIALS AND METHODS
Animals
A total numbers of animals were 150 camels. Out Of 150 camels,70 animals showed signs and about 19 of them were dying within three days after the appearance of the first clinical signs.The age of animals ranged from 3 to 8 years.The diet was a variable mix of Rhodes grass or hay, barley, maize and dates. Camels graze in a nearby farm field 20 km., where a number of grasses wet at this time of the year and the temperature reaches 45°C and this is a very suitable environment for the presence of fungi, especially with the presence of excess moisture.
Blood samples
Blood samples were collected using EDTA for hematological examinations. Most hematological parameters were made using a Coulter counter, electronic analyzer (Vet scan 5 HM–ABaxis–USA).
Serum Samples
Serum samples were collected in plain containers and centrifuged. This sample was stored at –20 until analysis.Total protein, albumin, glucose, blood urea nitrogen (BUN), creatinine, calcium, phosphorous, cholesterol , bilirubin and uric acid and the activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyltransferase (GGT), lactate dehydrogenase (LDH), creatine kinase (CK) and alkaline phosphatase (AP).These parameters were tested by coulter ELLEPC (Italy 2003).
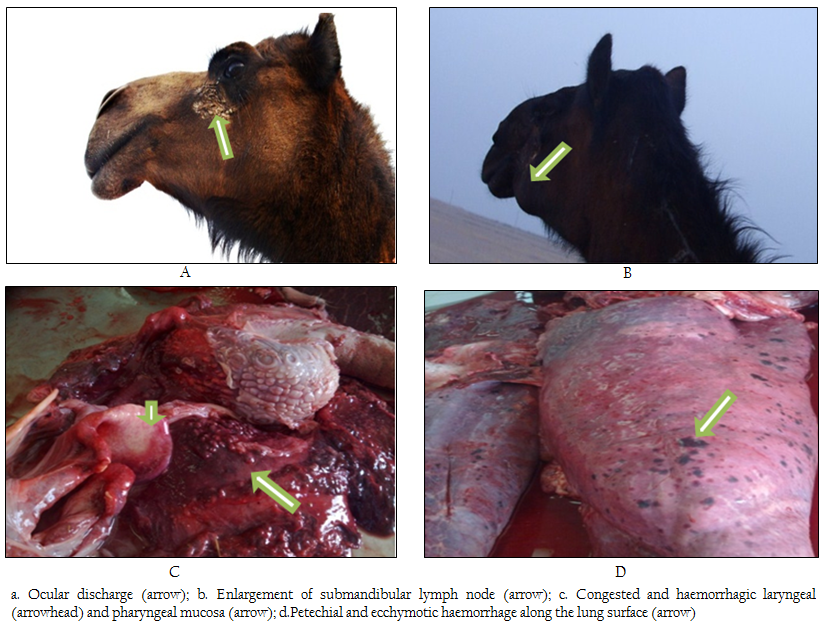
Figure 1: a. Ocular discharge (arrow); b. Enlargement of submandibular lymph node (arrow); c. Congested and haemorrhagic laryngeal (arrowhead) and pharyngeal mucosa (arrow); d.Petechial and ecchymotic haemorrhage along the lung surface (arrow)
Mycotic culture
At necropsy swabs taken from trachea, lung, omasum and intesine were inoculate into Sabouraud glucose agar plates containing chloramphenicol and actidione, and incubated at 37°C for 24 h and then incubated at 25°C for 3 successive days. Characteristics fungal growth was noted, mounted and examined microscopically (Scott, 1990).
Feedstuffs Analysis for Aflatoxin
The foodstuffs were collected and analyzed for aflatoxin. The samples were analyzed according to Trucksess et al. (1991).
Histopathology
Tissue specimens from lung, omasum, abomasum, small and large intestine, liver, and kidney as well as submandibular, bronchial and mesenteric lymph nodes were fixed in 10% formal saline and embedded in paraffin. Sections were stained with HE and Gomori methenamine silver stain for fungus demonstration (GMS).
RESULTS
Clinical Signs
The clinical signs displayed by affected animals were dyspnea, dullness and progressive weight loss. The oral mucosa was congested. Nasal and ocular discharges were noticed in the majority of cases (figure 1a).
In several cases, there was swelling of the throat and associated with enlargement of submandibular lymph nodes (figure1b). Epistaxis was noted in some animals associated with bloody diarrhea in most cases. Pyrexia with an increase in heart and respiratory rates. The nervous signs were hyperexcitability, marked incoordination, Sternal recumbency proceeded to lateral recumbency, inability to stand, convulsions, and death within 3–5 days.
Clinical Pathology and Chemistry Findings
The persistent hematological finding has been an often profound leucopenia with white cell counts ranging from 1.3±0.5 × 103 (normal value 14.4±5× 103).The most clinical chemistry changes were increases in AST reached to 83.2±36.6IU\L (normal value 64.16±8.13 IU\L).
Toxicological Findings
There was no evidence for the presence of aflatoxins in the feedstuffs.
Necropsy Findings
The most striking necropsy findings were hemorrhage in the most organs. Oedema and hemorrhages were present in the throat, submandibular, pharyngeal and laryngeal regions (figure 1c). Hemorrhages were always observed on the mucosa of the trachea and accompanied by frothy exudates.

Figure 2: : a. Ecchymotic haemorrhages along the mucosal surface of abomasum (arrow); b. Liver showing massive areas of haemorrhage (arrow). HE x400; c. lung showing congested blood vessels (arrowhead) and perivascular cuffing (arrow). HE x400.
The surface of the lungs displayed large numbers of petechial and ecchymotic hemorrhages (figure 1d). Lymph nodes of the thoracic cavity were oedematous and hemorrhagic. Epicardium and endocardium hemorrhages were also noticed in some cases. The mucous membranes of omasum and abomasum were severely congested and hemorrhagic (figure 2a). Massive hemorrhages and clots obstruct the small intestine and may extend to cecum. Associated lymph nodes were severely congested and oedematous. Few cases had black–stained feces.
Mycotic Growth
Cultures from trachea, lung, omasum and intestine resulted in growing colonies within 1 to 3 days. The colonies were identical with that of A.fumigatus as described by Pickett et al., (1985).
Microscopic Lesions
The tracheal and bronchial mucosa revealed fibrinonecrotic membrane associated with vascular thrombosis of submucosal blood vessels. This finding was accompanied by lymphocytic and histolytic aggregations. Alternative areas of necrosis could be seen everywhere in lung tissue. In the submandibular, bronchial and mesenteric lymph nodes the subscapular sinuses showed marked lymphocytic aggregations associated with prominent vascular congestion. The most striking lesions in omasum, small intestine and colon were fibrinonecrotizing inflammation accompanied by capillaries thrombosis with lymphocytic and histocytic aggregations in the submucosa.In the liver, there were multiple necrotic areas of various sizes, most of which were located in the portal area. This was together with massive areas of hemorrhages (figure 2b). Moreover, the blood vessels of lung, liver and kidney revealed severely congested blood vessels and perivascular cuffing (figure 2c). In some cases angioinvasion by Aspergillus fumigatus was evident.
DISCUSSION
Aspergillus fumigatus is a public feed contaminant particularly ubiquitous in preserving forages, produces some mycotoxins that can affect the animal health.The clinical findings were characterized by fever, lachrymation, swelling of the throat, and bloody diarrhea. Nervous signs and epistaxis proceeded in death were also recorded. These findings were consistent with the findings of other researches (Jubb et al., 1993; Mandal and Gupta, 1993).Leucopenia and increased AST activity was prominent in most cases. This finding was agreed with Muntz (1999) in an aging lactating alpaca (Lama Pacos).The decrease in the percentages of leucopenia may be due to suppression of lymphocyte blastogenesis by A. fumigatus (Chaparas et al., 1986).
The most salient lesions were observed in lung, liver, omasum, abomasum, small and large intestine, and the associated lymph nodes. This indicates the generalized infection. This infection may be developed from the embedding of spores on the naso–pharynx mucosa and lower respiratory passages, and the granulomatous lesions are somewhat slow to develop and so not seen in acute cases, hence the dissemination of the fungus from the pulmonary lesions can occur (Jubb et al., 1993). These findings were concomitant with Abbas and Omer (2005) who found out that A. fumigatus was incriminated in an outbreak of bronchopneumonia and gastroenteritis that affected a large number of racing camels in the Emirates.Several authors confirmed these findings in horses, cattle and lambs (Jungerman and Schwartzman, 1972; Sarfati, 1996). Furthermore, A.fumigatus is considered to be the fungal agent that associated with systemic diseases in dromedary camels (Boudra and Morgavi, 2005). The most outstanding necropsy findings were hemorrhage of the most organs, lung, liver, omasum, abomasum, intestine and kidney.This unusual coagulopathy may be due to some enzymes elaborated by Aspergillus sp. (McClellan et al.,1985). Furthermore, there are some suggestions that the ingestion of moldy food infected with A. fumigatus can cause fatal hemorrhagic disease in cattle and swine (Jubb et al., 1993).
Histopathological changes were numerous foci of necrosis in the lungs and liver as well as fibrinonecrotizing inflammation in omasum, small intestine and colon associated with vascular thrombosis and hemorrhages. Such lesions could be attributed to the invasion of Aspergillus organisms into the vascular system and consequent thrombosis of small to medium sized vessels associated with ischaemic necrosis (SEVERO, 1989).
In conclusion, according to our results, A. fumigatus should be considered among the diseases of the fatal and invasive nature among camels.
ACKNOWLEDGEMENT
The authors are grateful to the Deanship of Scientific Research, King Faisal University, Saudi Arabia for the financial support.
REFERENCES
Abbas B, Omer OH (2005). Review of infectious diseases of the camel. Vet. Bulletin. 75(8): 1N – 16N.
Bhatia KC, Kulshreshtha RC, Paul GuptaRK (1983). Pulmonary aspergllosis in camel. Haryuna Vet. 22: 118 – 119.
Boudra H, Morgavi DP (2005). Mycotoxin risk evaluation in feeds contaminated by Aspergillus fumigatus. Anim. F. Sci. Tech. 120: 113 – 123.
http://dx.doi.org/10.1016/j.anifeedsci.2005.01.006
Chaparas SD, Morgan PA, Holobaugh P (1986). Inhibition of Cellular Immunity by products of Aspergillus fumigatus. J. Med. Vet. Mycol. 24: 67 – 76.
http://dx.doi.org/10.1080/02681218680000081
PMid:3517279
EL–khouly AB, Gadir FA, Cluer DD, Manefield GW (1992). Aspergillosis in camels affected with a specific respiratory and enteric syndrome. Aus. Vet. J. 69 (8): 182 – 6
http://dx.doi.org/10.1111/j.1751-0813.1992.tb07515.x
PMid:1530552
Gareis M, Wernery U (1994). Determination of Gliotoxin in samples associated with cases of intoxication in camels. Mycotoxin Res. (10): 2 – 8.
Jubb KVF, Kennedy PC, Palmer's N (1993). Pathology of Domestic Animals, 4th edn. Academic Press,San Diego.
Jungerman PF, Schwartvnan RM (1972). Vet. Med. Myco. Lea and Febiger, Philadelphia. p 75.
Kwon–Chung KJ, Bennett JE (1992). Med. Myco. Lea &Febiger, Philadelphia.
Khalid A AL–Busadah (2007).Some Biochemical and Haematological Indices in Different Breeds of Camels in Saudi Arabia. Sci. J. King Faisal Univ. (Basic and Applied Sciences) 8 (1):131.–.142.
Lacey J (1996). Spore dispersal– its role in ecology & disease: The British contribution of fungal aerobiology, Myco. Res. 100: 641 –660.
http://dx.doi.org/10.1016/S0953-7562(96)80194-8
Mandal PC, Gupta PP (1993). Experimental aspergillosis in Goats: Clinical, Haematological and Mycological Studies. J. Vet. Med. 40: 283 – 286.
http://dx.doi.org/10.1111/j.1439-0450.1993.tb00139.x
McClellan SL, Komorowski RA, Farmer SG, Hussey CV, Kauffman HM, Adams MB (1985). Severe bleeding diathesis associated with invasive aspergillosis in transplant patients. Transp. 39(4):406 – 10.
http://dx.doi.org/10.1097/00007890-198504000-00014
Muntz FH (1999). Oxalate–producing Pulmonary Aspergillosis in an Alpaca. Vet. Pathol. 36: 631 – 632.
http://dx.doi.org/10.1354/vp.36-6-631
PMid:10568451
Pickett JP, Moore CP, Beehler BA, Gendron FA, Dubielzig RR (1985). Bilateral chorioretinitis secondary to disseminated aspergillosis in an alpaca. J. Am. Vet. Med. Assoc. 187: 1241 – 1243.
PMid:4077653
SarfatiJ, Jensen HE, Latge JP (1996). Route of infections in bovine aspergillosis. Med. Myco. Vol. 34(6): 379–383
http://dx.doi.org/10.1080/02681219680000681
Scott BM (1990). Natwal Pohonr, AOAC. Washington DC, p 1191 Sheridan JJ (1981) Vet. Res. Commun 5:1 Oxford, p 301.
Severo LC, Bohrerjc, Geyergr, Ferreirol (1989). Invasive aspergillosis in an alpaca (Lama pacos). J. Med. Vet. Myco. 27: 193–195.
http://dx.doi.org/10.1080/02681218980000261
PMid:2778579
Taylor RT Dagenais, Nancy P Keller (2009). Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clinical Microbiogy Reviews 22(3): 447 – 465.
http://dx.doi.org/10.1128/CMR.00055-08
PMid:19597008 PMCid:PMC2708386
Trucksess MW, Stack ME, Nesheim S, Page SW, Albert RH, Hansen TJ (1991). Immunoaffinity column coupled with solution fluorometry or liquid chromatography post–column derivatization for determination of aflatoxins in corn, peanuts, pea nut butter: collaborative study. J. Assoc. Off. Anal. Chem. 74 (1): 81.
PMid:2026580





