Advances in Animal and Veterinary Sciences
Research Article
Antioxidant, Hepatoprotective and In vitro Cytotoxic Activities of Cichorium intybus L. Extract
Mohamed Atef1, Abo-Baker Y.I. El-Gendi1, Aziza M. Amer1, Bayan Abd Al Razzak2, Khaled Abo-EL-Sooud1*, Sherin I. Ibrahim1
1Pharmacology Department, Faculty of Veterinary Medicine, Cairo University, Egypt; 2Pharmacology Department, Faculty of Veterinary Medicine, Al-Baath University, Syria.
Abstract | The ethanolic extract of Cichorium intybus L was evaluated for its potential antioxidant, hepatoprotective in rats, and in vitro cytotoxic effects. Preliminary phytochemical screening of C. intybus extract revealed tannins, resin, saponins, alkaloids, sterols/triterpenes, flavonoids, and ketones/esters as secondary bioactive compounds. The LD50 of the extract was evaluated using different gradual doses in Swiss mice and the mortality rate were recorded. The hepatoprotective and antioxidant effects of the orally administered C. intybus extract for five consecutive days in doses of 400 and 800 mg/kg b.wt., respectively, were assessed in CCl4-intoxicated rats’ model via biochemical and histopathological findings. Finally, the in vitro cytotoxicity of the extract was evaluated against three tested human cell lines which are colon cancer (HCT 116), breast cancer (MCF-7) and, liver cancer (HEPG2) cell lines using sulforhodamine B assay (SRB). The extract was confirmed as non-toxic orally with a wide safety index in rodents. C. intybus extract is significantly ameliorated the hepatic antioxidant potency via increasing the reduced glutathione (GSH) and decreasing lipid peroxidation (LIP) values in a dose-dependent method. Furthermore, the histopathological picture of liver tissue and the serum biochemical parameters serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in CCl4-intoxicated rats are significantly recovered. The in vitro potential cytotoxicity of the extract showed potent cytotoxic activity against HCT 116, and HEPG2 cell lines. The hepatoprotective, antioxidant, and cytotoxic activities of C. intybus extract could be a promising alternative source for pharmaceutical industries.
Keywords | Cichorium intybus, Antioxidant, Hepatoprotective, Cytotoxicity.
Received | October 12, 2020; Accepted | October 20, 2020; Published | December 10. 2020
*Correspondence | Khaled Abo-El-Sooud, Pharmacology Department, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: kasooud@cu.edu.eg
Citation | Atef M, El-Gendi ABYI, Amer AM, Al Razzak BA, Abo-El-Sooud K, Ibrahim SI (2021). Antioxidant, hepatoprotective and in vitro cytotoxic activities of Cichorium intybus L. extract. Adv. Anim. Vet. Sci. 9(1): 137-142.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.137.142
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abo-El-Sooud et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Despite there are numerous hepatoprotective agents that have been used, there are no entirely effective treatments that ameliorate hepatic function with almost comprehensive safeguarding and rapid regeneration of hepatic cells (Chattopadhyay, 2003). Besides, some chemotherapeutics can induce adversative or untoward effects on hepatic biochemical and histological patterns (Shirani et al., 2017). Therefore, there is a worldwide necessity for effective and safe hepatoprotective agents. Natural crude extracts and isolated secondary bioactive botanical metabolites are the most alternative and promising sources. A lot of ethnobotanical chemical substances have been used worldwide with high efficacy as hepatoprotective, antiulcerogenic, anti-inflammatory, appetizer, stomachic, liver tonic, immunomodulator, and anticancer properties (Matos et al., 2018).
Cichorium intybus L. “Chicory” is an edible plant that belongs to the family Asteraceae, growing in Europe, India, and Egypt. The plant contains several medicinally important compounds such as coumarins, flavonoids, and vitamins and used in traditional medicine as an antihepatotoxic, anti-inflammatory, liver tonic, cholagogue, diuretic and also is used as a tonic and anticancer agent (Liu et al., 2013; Satmbekova et al., 2018).
C. intybus is a multi-benefits bioactive plant in different regions, with an efficient antiparasitic effect. The feeding chicory can reduce the shedding of parasitic egg counts in animals as chicory is identified to synthesize sesquiterpene lactones that induce potential antiprotozoal activity in animals and poultry (Peña-Espinoza et al., 2018). This approach assists to achieve dual purposes for overcoming the drug resistance and drug residues in products of animal origin sustainable natural products for controlling coccidiosis (Ramadan et al., 1997).
Approximately half of total cancer–related deaths among men and women in the western nations are breast, colon, and prostate carcinomas (Jemal et al., 2010). The use of radio and chemotherapy, and, surgery in cancer management, as evidenced by the high-risk assessment rates that indicate there is a necessity for an alternative therapeutic approach for the neoplastic plan. C. intybus has been assessed as an in vitro antiproliferative activity against prostate cancer (LNCaP), amelanotic melanoma (C32), and renal adenocarcinoma (ACHN) cell lines (Mohammad et al., 2014). Additionally, crude methanolic extract of chicory exerted a potent cytotoxic activity (90%) against MCF-7 and AML-7 cell lines at 500 µg/mL (Kandil et al., 2019).
Chicory extract is a promising remedy for arthritic gout through inhibiting the interleukin IL-1β release by suppressing nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) that induce inflammatory responses and cell apoptosis in response to risk signals (Wang et al., 2019). The block or converse of cancer etiology at the cellular and molecular stages involves the use of recently isolated bioactive botanical extracts (Mehta et al., 2010).
In this study, we assessed the hepatoprotective, antioxidant, and, in vitro cytotoxic against hepatic and colon cell lines activities of the ethanolic extract of C. intybus.
MATERIAL & METHODS
Plant Material
The aerial part of C. intybus L. “Chicory” plant was identified at the Flora division, ministry of Agriculture, Giza, Egypt. The plants were separated and air-dried away from the sun then powdered and kept in a tightly closed glass container at room temperature till the extraction time.
Five hundred grams of the dried plant was extracted by percolation with ethanol 75% using the Rota-vapor apparatus at 40°C till semisolid ethanolic extracts were obtained. The extract was kept refrigerated until used. The obtained extract was dissolved using a few drops of tween 80, then distilled water was added to prepare a solution with the desired concentration.
Preliminary Phytochemical Screening
The extracts were screened for the presence of different bioactive compounds by thin-layer chromatography (TLC) (Zarzycki, 2006). While the detection of saponins, tannins, glycosides/carbohydrates, and anthraquinones are carried out using anisaldehyde-sulphuric acid, ferric chloride, naphtho-resorcinol, and potassium hydroxide reagents respectively under ultraviolet light (Apak et al., 2016).
Animals
Twenty-five Female Wistar Albino rats weighing 170-200 g and aged between 7 to 8 weeks and thirty female Swiss mice (18–22 g) at age of 3-5 months were obtained from the Laboratory Animal unit, faculty of Veterinary Medicine, Cairo University. Experimental animals were housed in plastic cages, under restricted hygienic measures for 15 days before the study to be acclimatized. All animals were fed a balanced diet and water was provided ad libitum. The Institutional Animal Care and Use Committee (IACUC), Cairo University approved this study.
Determination of LD50
LD50 of the studied extract was determined by using four doses (0.5, 1, 2.5 and, 5g/kg). Thirty female Swiss mice (20-25 g) were allocated randomly into 5 groups of five each and were placed in separated observation cages. The symptoms of discomfort and the mortality rate were recorded for 72 hours after oral administration (Tanira et al., 1996).
Hepatoprotective and Antioxidant Effects
Twenty-five female rats were assigned randomly into 5 groups of five rats each. Group I was kept as control negative rats were given (vehicles) 1 mL of 0.5% Tween 80 in water orally and olive oil [1 mL/kg b.wt. subcutaneous injection (SC)] at days 2 and 3. Group II (CCl4-intoxicated group) were given a single daily dose of 1 mL of 0.5% Tween 80 in water orally and on the 2nd and 3rd days were administered SC (2 mL/kg b.wt.) CCl4:olive oil (1:1). Group III and IV animals were orally administered ethanolic extract of C. intybus in doses of (400 and 800 mg/kg b.wt.), respectively, and a single dose of CCl4 (2 mL/kg b.wt. SC, on days 2 and 3), 30 min after extract administration. Group V animals were orally administered silymarin, (as a standard group) at a dose of 100 mg/kg b.wt. and a single dose of CCl4 (2 mL/kg b.wt. SC, on days 2 and 3), 30 min after silymarin administration (Suja et al., 2004). All the oral dosages were administered daily for five consecutive days.
Table 1: Hepatoprotective effect of C. intybus L ethanolic extract in rat’s liver homogenate. (n = 5, Mean ± S.D.)
| Group | Dose mg/kg b.wt. |
ALT U/mL |
AST U/mL |
ALP U/mL |
Protein g/dL |
Albumin g/dL |
| Control | Solvent |
33.75±1.92a |
162.00±1.41a |
200.00±14.14a |
6.55±0.31a |
3.55±00.18a |
| CCl4-intoxicated | 2 mL |
60.50±1.12b |
225.25±2.64b |
460.00±6.10b |
5.75±0.08a |
3.23±0.31a |
| C. intybus | 400 |
47.25±1.30a |
198.00±7.21a |
274.50±2.57a |
5.97±0.33a |
3.30±0.23a |
| C. intybus | 800 |
46.75±1.30a |
190.75±3.19a |
263.20±10.96a |
6.03±0.28a |
3.43±0.18a |
| Silymarin | 100 |
40.8±1.26a |
177.75±2.22a |
228.00±17.2a |
6.10±0.17a |
3.50±0.12a |
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
Sampling
Blood samples were collected from the eye canthus of each rat 24 h after the last doses for assessing the biochemical parameters AST, ALT and ALP, total protein and, albumin.
Liver tissue samples were collected and divided into 2 parts, first part (fixed in 10% buffered formalin solution) for histopathological investigation. Another part was kept at – 80°C until the assessment of the antioxidant activity of reduced glutathione level (GSH) and Lipid peroxidation activity (Malondialdehyde) in the liver homogenate.
Measurement of potential Cytotoxicity sulforhodamine- B assay (SRB)
This work was carried out at National Cancer Institute Cairo-Egypt. The potential cytotoxicity of the extract was evaluated against three tested human cell lines which are colon cancer cell line (HCT 116), breast cancer cell line (MCF-7) and, liver cancer cell line (HEPG2). The cells were plated in 96 multi-well plates (104 cells/well) for 24 h before treatment with the compound to allow attachment of cells to the wall of the plates. Different concentrations of the tested extract (0, 1, 2.5, 5 and, 10 µg/mL) were added to the cell monolayer triplicate wells were prepared for each dose. The relation between the surviving segment and drug concentration is plotted according to El-Awady et al. (2016).
Statistical Analysis
Results are expressed as mean ± standard deviation (SD) and were evaluated for significance using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test (P ≥ 0.05).
RESULTS
Phytochemical Screening of C. intybus L ethanol extract
Preliminary phytochemical screening of C. intybus extract revealed the presence of tannins, resin, saponins, alkaloids, carbohydrate, sterols/triterpenes, flavonoids, and ketones /esters. No anthraquinones or amino acids were determined.
Acute Toxicity
Oral administration of tested extract in mice in doses up to 5 g/kg didn’t cause any signs of toxicity and deaths up to 72 h post-administration.
Hepatoprotective Effect
The result showed that CCl4 intoxication significantly increased the ALT, AST, and ALP levels when compared to normal rats, respectively. The ethanolic extract of C. intybus (400 and 800 mg/kg b.wt.) and silymarin significantly saved the liver and their transaminases and ALP, respectively. There are no alterations in protein and albumin concentrations in all groups. (Table 1).
Hepatic Antioxidant Levels
The ethanolic extract of C. intybus (400 and 800 mg/kg b.wt.) significantly improved the antioxidant activity in liver homogenate by increasing the levels of reduced glutathione (GSH) in CCl4-intoxicated group, respectively. Additionally, the levels of lipids peroxidation (LIP) were significantly decreased in C. intybus-treated rats (Table 2).
In Vitro Cytotoxicity By Sulforhodamine B
Assay (Srb)
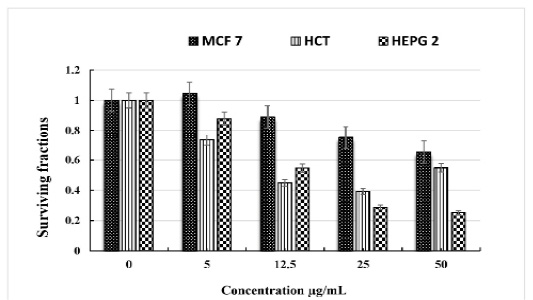
The obtained data showed that C. intybus extract showed potent cytotoxicity against HCT 116, and HEPG 2cell lines with IC50 values of 14.4 and 10.8 µg/mL, respectively. The extract has a weak cytotoxic effect on MCF 7. (Table 3 & Figure 1).
Histopathological Findings
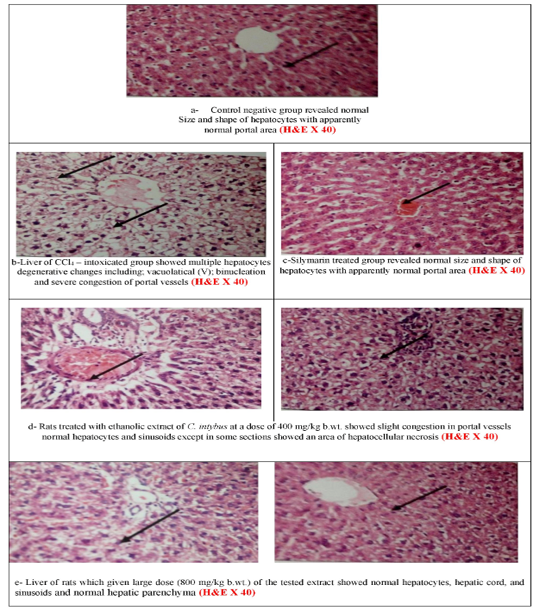
Microscopically, the liver of CCl4–intoxicated group showed multiple degenerative changes including; vacuolatical, binucleation, and severe congestion of portal vessels. Rats treated with ethanolic extract of C. intybus at a dose of 400 mg/kg b.wt. showed normal hepatocytes with slight congestion. While at the higher dose, normal hepatocytes, hepatic cord, and sinusoids as well as showing normal in the portal area. The negative control and silymarin-treated groups revealed normal size and shape of hepatocytes with
Table 2: Hepatic antioxidant effect of ethanolic extract of C. intybus in rat’s liver homogenate. (n = 5, Mean ± S.D)
| Parameters | Control negative | CCl4-Intoxicated group | Silymarin 100 mg/kg |
C. intybus 400 mg/kg |
C. intybus 800 mg/kg |
| GSH nmmol/g. tissue |
34.60±1.62a |
2.85±0.91b |
22.38±1.69a |
6.06±0.37b |
12.86±1.71a |
| Lipid peroxidation nmmol/g. tissue |
7.52±1.19a |
24.35±2.45b |
12.55±0.94a |
16.07±3.49a |
16.80±1.15a |
Means with different superscripts (a and b) within the same column are significantly different at P value ≤ 0.05
Table 3: The in vitro cytotoxicity of C. intybus L ethanolic extract on different cell lines.
| Concentration µg/mL | Breast cancer cell MCF 7 | Colon cancer cell HCT | Liver cancer cell HEPG 2 |
| 0 | 1 | 1 | 1 |
| 5 | 1.046 ± 0.43 | 0.734 ± 0.26 | 0.879 ± 0.35 |
| 12.5 | 0.89 ± 0.21 | 0.449 ± 0.16 | 0.547 ± 0.13 |
| 25 | 0.751 ± 0.14 | 0.392 ± 0.08 | 0.288 ± 0.07 |
| 50 | 0.653 ± 0.11 | 0.548 ± 0.10 | 0.254 ± 0.05 |
the normal portal area (Figures 2a-e).
DISCUSSION
The safety of the ethanolic extract of C. intybus was shown by the high LD50 value (>5g/kg) without any alteration in behaviors and activity of mice. The present data revealed potent hepatoprotective and antioxidant effects of oral administration of C. intybus ethanolic extract. The hepatoprotective efficacy is dependent on its capacity to sustain the normal physiological function of hepatocytes that were exposed to CCl4 toxicity. This agent induces hepatic injury with subsequent release of reactive metabolites of CCl4 such as trichloromethyl radical (CCl3) and trichloroperoxyl radical (CCl3O3) (Cachón et al., 2017). Additionally, these free radicals cause oxidative stress with significantly increasing serum ALT, AST, ALP, and liver lipid peroxidation while decreasing the activities of hepatic reduced glutathione (Rasool et al., 2014). Our result showed that oral administration of extract had antioxidant activity in CCl4 intoxicated groups that indicated by increasing in reduced glutathione (GSH) and decreasing in Lipid peroxidation (LIP). These findings are in agreement with Saeed et al. (2017), who suggested that C. intybus may be used for nutritional and pharmaceutical purposes in livestock. They added that the plant plays a key role as an antioxidant with a chief protective of hepatocytes without inducing therapeutic adverse effect by modifying the lipid peroxidation patterns in blood and hepatic tissues. Also, Abbas et al. (2015) reported that the hydroalcoholic extract of C. intybus possesses marked antioxidant properties and has significant protection against oxidative radicals and nucleic acid damage which could be attributed to the presence of polyphenols flavonoid which has hydrogen donating ability against an oxidative injury. Our result showed also that Cichorium intybus extract has a hepatoprotective activity that was indicated by decreasing the level of liver enzymes (ALT, AST, and ALP). Ahmed et al. (2003) reported that hydroalcoholic extract of C. intybus leaves exhibited a significant protective effect against CCl4 induced hepatotoxicity. Our findings are also agreed with Atta et al. (2010) who suggested that methanol extract of chicory significantly restored the CCl4–induced alterations in biochemical parameters and cellular elements of blood. The hepatoprotective activity of C. intybus may be attributed to various mechanisms such as inhibition of mitochondrial enzymes responsible for the metabolism of CCl4 to highly reactive metabolites and the potent scavenging activity against free radicals’ species responsible for cell damage.
The present study demonstrated also the anticancer activity of C. intybus and showed that extract had potent cytotoxic activity against the colon cancer cell line (HCT 116), breast cancer cell line (MCF-7), and liver cancer cell line (HEPG-2). These findings were supported by Imam et al. (2019) who identified many metabolite constituents including anthocyanin, delphinidin,3,4dihydroxyphenethyl, and other novel phenolic compounds.
Additionally, Conforti et al. (2008); Pool-Zobel et al. (2002) reported that C. intybus extract had an antiproliferative effect in amelanotic melanoma and the aqueous extract of had a talented effect in colorectal, breast, and prostate tumors (Shaikh et al., 2014). Finally, Mohammad et al. (2014) described that C. intybus presented an excellent cytotoxic activity against Jurkat cells, a human leukemia cell line. Our results can be explained by findings reported by (Pool-Zobel et al., 2002) who stated that inulin has significant antineoplastic potency by diminishing azoxymethane that induced atypical lesions and colon neoplasia tumors. Another theory suggested that the extract of C. intybus had an anticarcinogenic effect due to the highest amount of phenolics (Imam et al., 2019).
CONCLUSION
The hepatoprotective, antioxidant, and cytotoxic activities of C. intybus extract could be a promising alternative source for pharmaceutical industries. Consequently, the results of the existing trials recommend the use of C. intybus extracts or products as a dietary supplement, especially in hepatic and carcinogenic crises. Further studies are necessary to elucidate the exact bioactive compound(s) that responsible for those pharmacological effects and to elucidate the exact method of antioxidant and cytotoxic effects.
acknowledements
The authors express sincere thanks to department of Pharmacology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
authors contribution
All authors contributed equally to the manuscript.
REFERENCES