Advances in Animal and Veterinary Sciences
Review Article
Bacillus cereus: Pathogenicity, Viability and Adaptation
Ahlam A. Gharib1, Marwa I. Abd El-Hamid1, Norhan K. Abd El-Aziz1*, Engy Y. Yonan1, Mai O. Allam2
1Microbiology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Sharkia, Egypt, 44511; 2Zagazig Veterinary Organization, Ministry of Agriculture, Zagazig, Sharkia, Egypt.
Abstract | Bacillus cereus (B. cereus) is a motile spore-forming Gram-positive bacterium. The endospores are broadly oval-shaped or sometimes cylindrical or round and are resistant to unsuitable conditions. The bacterium is a natural inhabitant in soil and has frequently been detected in various foods. Food contamination may occur during processing as B. cereus spores have strong adhesion properties through their biofilm formation. Two main types of an intestinal illness named emetic (vomiting) and diarrhea are caused by B. cereus. Moreover, the bacterium causes several systemic and local infections in both immunocompromised and immunocompetent individuals. These include fulminant sepsis, progressive pneumonia-like anthrax, and devastating infections of the central nervous system. The pathogenicity is intimately associated with tissue-destructive exo-enzymes production, such as hemolysins, phospholipases, proteases and the emetic toxin. B. cereus growth could be inhibited by high temperature (> 105°C) and canning and storage below 4°C. However, exposure of B. cereus to different stresses might result in an enhanced thermo-tolerance. We reviewed virulence attributes, pathogenesis of B. cereus, viability, and adaptation of this particular bacterium.
Keywords | Bacillus cereus, Foodborne infection, Virulence attributes, Heat stress
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Norhan K Abd El-Aziz, Assistant Professor of Microbiology, Faculty of Veterinary Medicine, Zagazig University, Sharkia, Egypt: Email: norhan_vet@hotmail.com, nourhan_vet@zu.edu.eg
Citation | Gharib AA, El-Hamid MIA, El-Aziz NKA, Yonan EY, Allam MO (2020). Bacillus cereus: Pathogenicity, viability and adaptation. Adv. Anim. Vet. Sci. 8(s1): 34-40.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.34.40
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Gharib et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A valid national food control system is necessary to preserve the health and safety of domestic or indigenous consumers (Whitehead, 1995). Bacillus cereus (B. cereus) is one of the primary foodborne bacterial pathogens. It is spore-forming, Gram-positive bacilli, (Kotiranta et al., 2000). Prevalent in a wide range of environments, the bacterium may exist in extensive categories of different dairy products and foodstuffs.
Bacillus cereus is a natural inhabitant in soil and has frequently been detected in various foods. Soil is the primary source of food contamination with B. cereus spores; however, additional contamination may occur during processing primarily due to its strong adhesion attributes. It causes two forms of food poisoning named emetic and diarrhea. The emetic (intoxication) form is induced by a preformed small cyclic peptide (cereulide). On the other hand, diarrhea (infection) form is caused by heat-labile enterotoxins formed in the small intestine during its vegetative growth. The emetic form is related to vomiting within 1-5 h after consumption of the contaminated foods. Meanwhile,the diarrhea form (watery diarrhea) is related to abdominal pain, and it occurs within 8-16 h after consumption of the contaminated food (Ehling-Shulz et al., 2005).
Bacillus cereus has developed adaptive systems that enable it to live and resist different stresses and the challenges of an altering environment. An initial mild heat can produce temporal resistance to the next heat treatment termed as thermo-tolerance. For instance, after preservation at non-lethal temperature (37 or 40°C) for several hours, an increased thermo-tolerance of mesophilic B. cereus at 50°C has been evidenced (Browne and Dowds, 2001).
Various proteins are induced and detected in a wide variety of bacteria during pre-exposure of the heat. For B. cereus, different mechanisms for the production of heat-induced proteins (HIP) and heat adaptation have been discussed (Mahakarnchanakul and Beuchat, 1999; Periago et al. 2002). This review focuses on the pathogenesis of B. cereus and their virulence attributes. Moreover, it discusses the adaption of B. cereus under stress conditions and provides insights into the control of its viability.
Bacillus species taxonomy and characteristics
The genus Bacillus is one of the largest and the most ubiquitous microorganisms worldwide. It is commonly found in the environment; however, only some species are known to cause infections in humans (Holt et al., 1994). The genus is known to have a maximum phenotypic heterogeneity and variations. Currently, it comprises 268 species and seven subspecies. The species are primarily divided into three groups based on the morphology of the sporangium and spore (Drobniewski, 1993; SMIs, 2018). Group 1 is further divided into large and small cell subgroups. Bacteria within the large cell subgroup produce central or terminal ellipsoidal or cylindrical spores that do not swell the sporangium. It includes Bacillus anthracis (B. anthracis), Bacillus cereus (B. cereus), Bacillus mycoides (B. mycoides), Bacillus megaterium (B. megaterium) and Bacillus thuringiensis (B. thuringiensis). Bacteria within the small cell subgroup include Bacillus pumilus (B. pumilus), Bacillus subtilis (B. subtilis), and Bacillus licheniformis (B. licheniformis). Group 2 contains variable ellipsoidal central spores and distended sporangia. It includes Bacillus circulans (B. ciculans) and Bacillus coagulans (B. coagulans). Bacillus brevis (B. brevis), Bacillus alvei (B. alvei) and Bacillus macerans (B. macerans) belonged to this group previously; nevertheless, they are now re-classified to another genus (Todar, 2012). Group 3 possesses variable bacilli that have swollen sporangia with terminal or sub-terminal spores. It includes Bacillus sphaericus (B. sphaericus). Recently, there has been a taxonomic development of the genus Bacillus in two selected groups named B. subtilis and B. cereus (SMIs, 2018).
Ecology of B. cereus
B. cereus is ubiquitous; therefore, its presence in most of the raw foods is considered inevitable. Soil is the primary source of food contamination with B. cereus spores. For example, similar genotypic strains were detected in milk and soil of dairy farms (Christiansson et al., 1999), as well as the soil and the corresponding cooked or chilled food of vegetable-origin (Guinebretière and Sanchis, 2003).
Further contamination may occur during processing mainly due to strong adhesion attributes of the bacterium. The spores may adhere to the surface of processing equipment and develop biofilms (Andersson et al., 1995). For example, the B. cereus strains in milk utensils can contaminate raw milk (Svensson et al., 2004). The bacterial strains sustain pasteurization and, therefore, drying equipment is also suspected to contaminate the pasteurized and powdered milk (Eneroth et al., 2001). This reveals a necessity for more hygienic applications to decrease processed food contamination (Guinebretière and Sanchis, 2003). Mill manufacturing and packaging substances, as additional sources of food contamination has also evidenced by B. cereus spores (Pirttijarvi et al., 2000).
Bacillus cereus virulence potential and its associated foodborne infection
Virulence attributes
For ready-to-eat foods, owing to its heat resistant endospores that can survive the cookery and manufacturing procedures, contamination with soil-borne B. cereus is an emerging health safety concern (Logan, 2012). Some B. cereus strains with heat-resistant endospores can cause intoxications shortly after consumption of contaminated food products, thus represent a serious health risk to consumers (Rajkovic et al., 2008).
The emetic (cereulide) toxin: Cereulide toxin is formed by growing cells of B. cereus (Paananen et al., 2002). It is a highly lipophilic potassium ion-selective ionophore (Teplova et al., 2006) that can be absorbed into the bloodstream from the gut (Paananen et al., 2002). Working as an ionophore cation that resembles valinomycin, the toxin is able to suppress the fatty acid oxidation, and subsequently, stop the mitochondrial activity (Mikkola et al., 1999). Induction of cellular damage (Shinagawa et al., 1996) and diabetes by causing beta cell dysfunctions (Vangoitsenhoven et al., 2014), and inhibition of the natural killer cells of the human immune system (Paananen et al., 2002) has been evidenced for the emetic toxin.
Diarrheal toxins (enterotoxins): There are at least five B. cereus enterotoxins that cause diarrhea. Two of them (hemolysin BL and non-hemolytic enterotoxins) are protein complexes. In contrast, the other three (enterotoxin FM, enterotoxin T, and cytotoxin K) are single protein substances (Beecher et al., 1995; Lund et al., 2000). Produced during vegetative growth in small intestine, the diarrheal form of food poisoning is induced by heat-labile enterotoxins such as hemolysin BL (HBL) encoded by hblABCD, non-hemolytic enterotoxin (NHE) encoded by nheABC, cytotoxin K (CytK), enterotoxin T and enterotoxin FM (Clair et al., 2010; Kim et al., 2015).
The enterotoxin FM, hemolysins, and degradative enzymes are not directly cytotoxic; nevertheless, they confer cytotoxic and hemolytic activities of B. cereus and its adhesion to epithelial cells (Luxananil et al., 2003; Van den Abbeele, 2012). The diarrheal enterotoxins are not labile at pH 4–11, and get deactivated at 56°C for 5 minutes (Jenson and Moir, 2003).
Haemolysin BL: It is a three-composite diarrheal hemolysin that includes two lytic parts (L2 and L1 encoded by hblD and hblC, respectively), and a binding protein B encoded by hblA (Lindbäck et al., 2004). Haemolysin BL also causes dermo-necrosis and vascular permeability resulting in fluid collection and concentration in ligated rabbit ileal loops. Therefore, in B. cereus diarrheal cases, this particular toxin has been considered a primary virulence factor (Beecher et al., 1995).
Non hemolytic enterotoxin: A non-hemolytic three-composite enterotoxin (NHE) has previously been categorized (Lund and Granum, 1996). It is represented by three genes of nheA, nheB and nheC, where each of these three parts is different from the HBL.
Cytotoxin K (hemolysin IV): It is a single protein (34 kDa) with the potential to cause necrosis and hemolysis. It causes severe food poisoning outbreaks (Lund et al., 2000).
Enterotoxin T: Identified as a single composite protein (41 kDa), the enterotoxin T (bceT) is associated with a lethal effect on mice after injection. Further to this, it has shown vascular permeability, Vero cell cytotoxicity, and fluid concentration in the ligated mouse ileal loop (Agata et al., 1995).
Enterotoxin FM: Evidence for the presence of enterotoxin FM was previously detected for B. cereus (Granum et al., 1996). This putative virulence factor was described to be involved in the bacterium shape, motility, adhesion to epithelial cells, biofilm formation, and vacuolization of macrophages. The gene encoding a putative cell wall peptidase (entFM) was identified by the Basal Local Alignment Search Tool (BLAST) (Tran et al., 2010).
Haemolysins
Haemolysin 1 (cereolysin): Cereolysin O (CLO, 55 kDa) is a thiol activated protein that combines with streptolysin-O. The protein causes hemolysis, and is considered lethal to mice upon injection. Haemolysin 1 is not heat stable, and subjects to inhibition by cholesterol and serum (Clair et al., 2010; Kim et al., 2015).
Haemolysin 2: Haemolysin 2 (hly II, 30 kDa) is not a heat-stable protein. It is not susceptible to cholesterol, but proteolytic enzymes. The hemolysin 2 protein’s toxicity has not yet been recognized in vivo (Jenson, 1997). It is hemolytic and cytotoxic to human cell lines and induces lysis of phagocytic cells. Its role in B. cereus-induced diarrhea has not been implicated. (Stenfors et al., 2008).
Phospholipases C
Bacillus cereus produces three phospholipases C. All phospholipases C have been cloned and are well characterized (Granum, 1994).
Phosphatidylinositol hydrolase (PI-PLC): Phosphatidylinositol hydrolase (PIH) hydrolyzes phosphatidylinositol (PI) and PI-glycan-containing membrane anchors. Both are essential structural components of one class of membrane proteins. The PIH is a 34 kDa protein, which is non-hemolytic and does not necessitate any ions for its biological action (Granum, 1994).
Phosphatidylcholine hydrolase (PC-PLC): Egg yolk (lecithin) hydrolysis is a significant characteristic for the identification of Bacillus spp. B. cereus group strains such as B. cereus, B. mycoides, and B. thuringiensis mostly possess a lecithinase activity (an egg yolk reaction). The reaction is stimulated by the phosphatidylcholine hydrolase (PCH), phospholipase C, which hydrolyzes lecithin (Schraft and Griffiths 1995).
Sphingomyelinase (SMase): Sphingomyelinase (SMase) is the enzyme that hydrolyzes sphingomyelin (SM) to ceramide and phosphocholine. When the enzyme attaches to membranes of sheep erythrocytes, it selectively hydrolyzes SM present in these membranes. The Ca2+ and Mn2+ions catalyze the attachment of the enzyme to these erythrocyte membranes, while Mg2+ ions stimulate their SM cleavage and hemolysis reaction (Tomiuk et al., 1998).
Regulation of B. cereus extracellular virulence factors: The transcriptional regulator, phospholipase C (plcR), mostly controls B. cereus virulence factors (Gohar et al., 2008) such as HBL, enterotoxin FM, PI-PLC, PC-PLC and SMase (Agaisse et al., 1999). Hence, this plcR appeared to be an essential virulence regulator for all B. cereus group categories. It binds to a nucleotide sequence called the PlcR box and subsequently activates the gene expression (Gohar et al., 2008) (Figure 1).
Bacillus cereus associated foodborne infection
Bacillus cereus is an emerging human foodborne pathogen (EFSA, 2009). Owing to the formation of Bacillus resistant endospores, all Bacillus species and related genera have long been troublesome to food producers. When infected food is not or inadequately cooked, Bacillus foodborne illnesses occurs due to the survival and vegetative growth of the bacterial endospores (Turnbull, 1996). If the cooking temperatures are less than or equal to 100 °C (212 °F), some B. cereus spores can survive (Roberts et al., 1996). Moreover, the storage of food at temperatures below 60 °C or inadequate cooling might support their growth in food following thermal treatment. Depending on the strain, growth to high levels may cause food spoilage and foodborne illness after ingestion (Setlow, 1994). Vegetative structures of the B. cereus do not tolerate environmental stresses such as heat, chemicals, or radiation. However, its spores are highly resistant due to their tough physical nature and metabolic latency. Consisted of several proteins that play a significant role in attachment of the spore to the surfaces, the external layer is called an exosporium (Stewart, 2015). Compared to vegetative cells, some spores might produce diarrheal disease, since they are better armed to pass through the gastric juice (Clavel et al., 2004). B. cereus is related to a few foodborne illnesses (2–5%) such as severe nausea, diarrhea, and vomiting (Kotiranta et al., 2000).
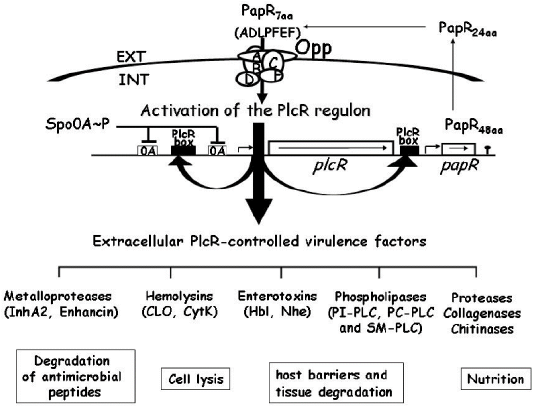
Figure 1: Diagrammatic representation of the transcriptional regulator PlcR and its cognate cell-cell signaling, peptide PapR (Gominet et al., 2001; Bouillaut et al., 2005; Gohar et al., 2008).
There are two forms of illness produced by B. cereus; diarrhea and emetic (vomiting) (Ehling-Shulz et al., 2005). The characteristics of these types of diseases are listed in Table 1. The first one is the emetic syndrome, which is characterized by nausea and diarrhea in nearly one-third of the cases. It occurs 1-5 h after the ingestion of contaminated-foods (Kramer et al., 1989). Emetic type is usually mild, but lethal cases have been evidenced rarely. Cooked rice, pasta, and milk supported important cereulide creation at 30°C, and its production below 10°C does not seem possible (Finlay et al., 2000). This clearly indicates that an occurrence and even growth of emetic strains of B. cereus do not reflect the production of cereulide in foods permanently (ICMSF, 2005). Diarrheal form of poisoning appears 8-16 h after ingestion of contaminated food and is accompanied by watery diarrhea and abdominal pain (Granum and Lund, 1997). It is commonly mild, but some fatal cases have been diagnosed due to bloody diarrhea (Lund et al., 2000). In general, it is recognized that the diarrheal form of poisoning occurs through enterotoxin production during the exponential phase of growth in the intestine (Granum, 1994). Enterotoxin is heat sensitive and gets deactivated at 56 °C (133 °F) for 5 minutes. Since the stomach enzymes do its degradation, following induction within the small intestine by surviving B. cereus spores may be the cause of the disease (Millar et al., 1998).
Bacillus cereus viability and adaptation to heat stress
Adaptation to heat stress
Production of a wide range of proteins has been evidenced in a large group of bacteria during the pre-exposure to heat. The mechanisms of heat-induced proteins (HIPs) production and heat adaptation of B. cereus have been previously discussed (Mahakarchanakul and Beuchat, 1999; Periago et al., 2002; Duport et al., 2016). The heat adaptation of B. cereus involved proteins (chaperones and proteases), which manage a variety of cellular functions including protective processes of protein folding, assembly, repair and prevention of aggregation under stress and non-stress conditions. During pre-exposure to 42°C, B. cereus was adapted to heat exposure at the lethal temperature of 50°C (maximum protection occurs after 15 min to 1 h of pre-exposure to 42°C) as a result of de novo protein synthesis. By using two-dimensional gel electrophoresis, Periago and his colleagues grouped the observed 31 heat-induced proteins into 3 groups on the basis of the time points of maximal production, then they determined the N-terminal sequences of a subset of these proteins (Periago et al., 2002). This revealed the induction of stress proteins (CspB, CspE, and SodA), proteins involved in sporulation (SpoVG and AldA) and metabolic enzymes (FolD and Dra), identified heat-induced proteins in related organisms (DnaK, GroEL, ClpP, RsbV, HSP16.4, YflT, PpiB, and TrxA), and other proteins (MreB, YloH, and YbbT) (Periago et al., 2002). The above mentioned authors also added that the up regulation of several stress proteins was confirmed by using antibodies specific for the well-characterized B. subtilis HSPs, except GroES. Finally, they similarly found that a 30 min pre-exposure to 4% ethanol, pH 5, or 2.5% NaCl also resulted in increased thermotolerance with the induction of some HSPs (Periago et al., 2002) suggesting that during mild processing, a cross-protection from heating occurred in pathogenic B. cereus, which may result in increased its survival in foods. The continuous production of the classical heat shock chaperones and protease upon heat exposure might be related to regulation by HrcA via CIRCE (controlling inverted repeat of chaperones expression) elements. Upstream of the gene encoding HrcA, a CIRCE element is found that exactly matches the conserved CIRCE sequence of B. subtilis. Because the upstream region of the groESL gene cluster is lacking in the B. cereus genome sequence, no
Table 1: Characteristics of the two disease syndromes caused by B. cereus (Lund et al., 2000; Clavel et al., 2004).
| Criteria | Diarrheal syndrome | Emetic syndrome |
| Infective dose |
105–107(cells g−1) |
105–108 (cells g−1) |
| Toxin produced | In the small intestine of the host | Preformed in foods |
| Type of toxin | Protein | Cyclic peptide |
| Incubation period | 8–16 h (occasionally >24 h) | 0.5–5 h |
| Duration of illness | 12–24 h (occasionally several days) | 6–24 h |
| Symptoms | Abdominal pain, watery diarrhea and occasionally nausea | Nausea, vomiting, and malaise (sometimes followed by diarrhea, due to additional enterotoxin production |
| Foods most frequently implicated | Meat products, soups, vegetables puddings/sauces, and milk/milk products | Fried and cooked rice, pasta, pastry and noodles |
CIRCE element could be identified upstream this operon (Periago et al., 2002).
Specific control for B. cereus viability
Sterilization is the most effective method for controlling B. cereus spores. Depending on the data of heat tolerance (Fernández et al., 1999), it is true that, the persistent exposure of heat ( 105º C for 3 min) can induce a 5 log decrease in the population of a highly resistant B. cereus strain. A higher temperature (> 105ºC) could defend any type of food from this bacterium. Nevertheless, only canning make sure a thorough damage of B. cereus spores. Usual cooking, non-lethal heat treatments of refrigerated food, and pasteurization are insufficient to destroy spores of B. cereus. The previous handling might stimulate bacterial spores to activate vegetation and cell growth. Hence, cooling and chilling at the earliest possible time, followed by storage at a refrigeration temperature are necessary to evade the growth of vegetative bacterial cells to a number that ensures a hazard to the safety of the product. When refrigeration is the primary way for controlling B. cereus growth in foods, a shallow temperature is much necessary. Only preservation under 4°C might confirm a lack of growth of B. cereus. An increase in the B. cereus concentration is dangerous when the storing temperature is elevated about 2º C, from 6º C to 8º C. On the other hand, a minor decrease in the water activity and pH could be adequate to inhibit B. cereus growth at refrigeration temperatures (Andersson et al., 1995). For instance, at 8°C, no increase was detected for three B. cereus strains at pH 6.5, while it happened after four lag time days at pH 7 (Benedict et al., 1993).
Other factors, such as the food nature, also impacts the refrigeration effect. For example, at 7 °C and similar pH, B. cereus strains that did not grow in the vegetable broth will be able to grow in the laboratory media (Choma et al., 2000). Therefore, in some food items, refrigeration could be more effective in retarding or inhibiting the growth of B. cereus. However, if the food content and storing circumstances inhibit B. cereus growth, a little original cell concentration remains vital and significant to be maintained. Consequently, proper cleaning and disinfection (for instance, hypochlorite at pH< 8) are essential to prevent high levels of B. cereus in food products. Well-designed pipelines to improve the cleaning process can fight against the formation of bacterial biofilms.
Conclusion
Bacillus cereus has been detected in almost all the categories of foodstuffs. It is frequently present as spores in the environment. Use of raw materials in complex foods or storing of the processed foods with circumstances appropriate for B. cereus will lead to the growth of B. cereus to a concentration that could be harmful to customers. B. cereus is concomitant mainly with food poisoning with two different forms, the emetic, and diarrhea. Additionally, systemic and local infections due to B. cereus may occur in immunocompromised patients. Bacillus cereus can survive under stress conditions, where an initial dose of non- lethal heat or any stress can produce heat-induced proteins and, subsequently, thermos-tolerance. Therefore, a suitable dualism of both the storage temperature and time for preventing of B. cereus growth is the main control application.
Authors Contribution
AAG, MIA and NKA made equal contributions to the conception and the design of the review. EYY and MOA performed the literature search and prepared the manuscript. All the authors contributed in writing of the manuscript, revised it critically for important intellectual contents and gave the final approval of the version to be published.
Conflict of interest
The authors have declared no conflict of interest.
References






