Advances in Animal and Veterinary Sciences
Research Article
Prevalence and Risk Factors of Foot and Mouth Disease Virus in Nineveh Province, Iraq
Saadoon Abdul-Satar Salim1, Al-Obaidi Qaes Talb2*, Albaroodi Safwan Yousif3, Hasan Sadam Daher2
1Department of animal production, College of Agriculture and Forestry, University of Mosul, Mosul, Iraq; 2Department of Internal and preventive medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; 3Department of Microbiology, College of Veterinary Medicine, University of Mosul, Mosul, Iraq.
Abstract | The objectives of the present research are to evaluate the prevalence of foot and mouth disease (FMD) in cattle employing competitive enzyme-linked immunosorbent assay (c-ELISA) and conventional reverse transcriptase polymerase chain reaction (RT-PCR technique), to identify FMDV genotypes and to investigate some epidemiological risk factors related to the disease prevalence. A total of 460 saliva swabs and blood samples were collected from cattle of different ages and sexes in Nineveh province, Iraq. Epidemiological data were collected through interviews with the farmer s’ employer. Viral RNA was extracted from saliva, and RT-PCR was conducted to identify the virus genotypes based on VP1 and VP3 genes amplification. The overall occurrence of FMD in Nineveh province based on c-ELISA test and RT-PCR was 46.95% and 40.43%, respectively. According to Kappa value (0.81), it has a mean that is of higher compatibility between c-ELISA test and RT-PCR for diagnosis of FMDV in cattle. The risk factors that are significantly associated with higher prevalence of FMD including: Feedlot calves >6 months – 2 years old, females, pregnant animals, imported animals, indoor feeding, large herd size, beef purpose, non-vaccinated, in north-eastern parts of the province and in spring and winter months. Four genotypes of FMDV (A, O, Asia1 and SAT) were detected in Nineveh province at (12.39%, 6.95%, 17.39% and 3.69%) respectively. Genotype C was not detected in this research. In conclusion, our results reported the first time detection of four FMDV genotypes in Nineveh province and there are several risk factors linked to the disease occurrence, which require strict control measures.
Keywords | FMD, Prevalence, Risk factors, c-ELISA, RT-PCR.
Received | June 25, 2019; Accepted | August 19, 2019; Published | December 08, 2019
*Correspondence | Al-Obaidi Qaes Talb, Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Mosul, Iraq; Email: qaes_talb@yahoo.com
Citation | Salim SAS, Talb OQ, Yousif AS, Daher HS (2020). Prevalence and risk factors of foot and mouth disease virus in nineveh province, iraq. Adv. Anim. Vet. Sci. 8(1): 1-10.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.1.1.10
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Talb et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Foot and mouth disease (FMD) has been described as “a highly contagious disease of cloven footed animals.” It is classified as a list A disease by OIE (2004), which is the most dreaded animal disease in the developed world due to very substantial economic losses in high producing animals by causing loss of production, costs of eradication and through the interference with movement of animals and meat between countries (Depa et al., 2012; Constable et al., 2017).
FMD virus belongs to the Aphthovirus genus of the family Picornaviridae, contains a single strand RNA genome (Longjam and Tayo, 2011). The FMD virus has seven major variable serotypes: A, O, C, Southern African Territories SAT-1, SAT-2, SAT-3 and Asia-1 (Sumption et al., 2012). There are over 60 subtypes with variable antigenicity and different degrees of virulence without cross immunity between serotypes and this causes difficulties for vaccination and control programs (Ringa and Bauch, 2014; Roche et al., 2015). The susceptibility of cattle to FMD infection depends on many risk factors such as species, gender, age, breed, region, management (indoor or outdoor), herd size, season, and climatic factors (Roche et al., 2015).
The main clinical manifestations of FMD infection depend on the viral serotypes, virulence of the virus, animal breeds and immune status of animals. The clinical signs of the disease are initiated by high fever (40℃ – 41℃) accompanied by anorexia, which is followed by painful stomatitis as well as formation of vesicles in the mouth, teats, and on the feet (Constable et al., 2017; Mansour et al., 2018). There is copious salivation and the saliva hangs in long rope-like strings (Bhattacharya et al., 2005; Knight et al., 2016).
The rupture of vesicles in the foot causes lameness and often recumbency with painful swelling of the coronet (Constable et al., 2017). The vesicles may appear on the teats and when the teat orifice s infected, severe mastitis often follows (Saraiva, 2004; Wadsworth et al., 2006). Infected pregnant animals may abort or may have stillbirths, with rapid loss of condition and decrease in milk yield occurs in the acute stage of the disease (Bulman and Terrazas, 1976). The mortality in adult animals is in general low, but it may be high in young animals due to acute myocarditis (Ramanon, 2016).
There are several specific ELISAs for detecting the non-structural proteins (NSP) of FMDV such as Mab trapping (MAT) ELISA and blocking ELISA for detecting of antibodies against 3ABC-NSP (Sorensen et al., 1998). Currently, competitive- ELISA is used for detecting NSP-FMD antibodies and this serological test is more sensitive and rapid than the virus neutralization test (Sevik and Ozturk, 2013). In addition, RT-PCR technique has become established as a confirmatory test. It is a very sensitive and rapid test used for diagnosis of FMDV (Paixão et al., 2008), also useful for typing FMDV isolates and selecting the suitable emergency vaccine (Giridharan et al., 2005).
The studies of FMD and its risk factors are scanty in Nineveh province, therefore, the objectives of this research were to estimate the prevalence of FMD in cattle employing competitive enzyme-linked immunosorbent assay (c-ELISA test) and conventional reverse transcriptase polymerase chain reaction (RT- PCR technique), to determine FMDV genotypes in Nineveh province and to investigate some epidemiological risk parameters linked with the occurrence of the disease.
MATERIALS AND METHODS
Animals and Sample Collection
The study was carried out on 460 animals most of them apparently diseased, from both sexes and different ages (238 adult cows, 74 younger calves, 148 beeflot calves), reared in different parts of Nineveh province, Iraq (Western, South, Central, North, and Eastern parts of Nineveh province). The study started in September 2017 and ended in September 2018. Complete clinical examinations were done for all the animals and epidemiological data were collected through an interview with the farmer s’ owner. A total of 460 saliva swabs and blood samples were extracted from the jugular vein of cattle utilizing 18G needles in to sterile vacutainer tubes (5 ml each) without any anti-coagulant for serum separation. The collected samples were placed and taken to the laboratory in ice bags. The serum samples were kept at -20℃ until tested employing for c-ELISA technique. Saliva swabs were kept at -80℃ until used for conventional RT-PCR.
Competitive - Enzyme Linked Immunosorbent Assay (C- Elisa)
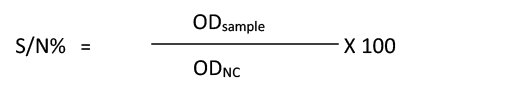
All 460 serum samples were analyzed using a commercial c- ELISA kit (IDvet, Grabels-France) which was used to detect anti-FMDV non-structural protein (NSP) antibodies in the serum of infected animals. The procedure was performed in compliance with manufacturer’s instructions of c-ELISA kit, and by using automatic plate reader (BioTek® Elx800, USA). For each sample, calculation of the competition percentage (S/N%) was as follows:
(OD = Optic density, NC = negative control)
The interpretation of the results includes: If samples presenting (S/N%) were less than or equal to 50% they were considered positive, while if they were greater than 50% they were considered negative.
Rna Extraction and Amplification from Cattle Saliva Swabs
The RNA of FMD virus was extracted from 460 saliva swabs employing the PrimePrepTM viral RNA extraction kit, complying with the manufacturer’s instructions (GeNet Bio, Korea). Amplify the highly conserved VP1 and VP3 genes of FMD virus from swabs (n=460), as a target in conventional RT-PCR technique using GeNet Bio OneStep RT-PCR Kit (GeNet Bio, Korea) for FMDV detection and determination of genotypes. In this study, the oligonucleotides primers include: universal ‘catch-all’ primers (1F and 1R) to detect all possible FMDV genotypes (Jamal et al., 2011; Al-Rodhan, 2014) and specific primers for detection of FMDV: genotype A (C612F and EUR-2B52R), genotype O (ARS4F and EUR-2B52-R) (Nick et al., 2005), genotype Asia-1 (As1-1C-505-F and
Table 1: The oligonucleotide primers used to amplify the VP1and VP3 genes.
| Genotypes | Primers | Sequences 5’-3’ | Expected size (bp) |
| Universal | 1F | GCCTGGTCTTTCCAGGTCT | 328 |
| 1R | CCAGTCCCCTTCTCAGATC | ||
| A | A-C612F | TACCAAATTACACACGGGAA | 865 |
| EUR-2B52R | GACATGTCCTCCTGCATCTGGTTGAT | ||
| O | ARS4F | ACCAACCTCCTTGATGTGGCT | 1301 |
| EUR-2B52R | GACATGTCCTCCTGCATCTGGTTGAT | ||
| Asia-1 | As1-1C-505-F | TACACTGCTTCTGACGTGGC | 911 |
| NK61-R | GACATGTCCTCCTGCATCTG | ||
| SAT | 1D209F | CCACATACTACTTTTGTGACCTGGA |
715–730
|
| 2B208R | ACAGCGGCCATGCACGACAG | ||
| C | C-1C536 | TACAGGGATGGGTCTGTGTGTACC |
833–877
|
| R-NK61 |
GAC ATG TCC TCC TGC ATC TG |
NK61-R) (Zinnah et al., 2012; Baba Sheikh et al., 2017), genotype SAT (1D209F and 2B208R) and genotype C (C-1C536 and R-NK61) (Reid et al., 2000; El-Bayoumy et al., 2014), (provided by Macrogen, Japan) (Table 1). The conventional RT-PCR reactions were conducted in a total volume of 20μl, composed of 6μl of dH2O, 1μl of each forward and reverse primers, 2μl of template (RNA sample) and 10μlof 2X SuPrimeScript RT–PCR Premix. The mixture was briefly centrifuged and reverse transcription was done at 50°C for 30 min in the thermo-cycler machine. This was followed by initial denaturation at 95°C for 5 min, then by 40 cycles of denaturation at 95°C for 30 sec, annealing (at 55°C for 30 sec to universal and Asia-1 genotype, to genotypes A and O, at 58°C for 30 sec and at 57°C for 30 sec to genotypes SAT and C), extension at 72°C for 1 min and final extension at 72 °C for 5 min. All amplicons were electrophoresed on a 1.5% agarose gel in TBE buffer and visualized under UV light.
Statistical Analysis
The variations in the occurrence between the several risk factors parameters were evaluated by employing two-sided Chi-square and Fischer’s exact test in IBM-SPSS statistics version19 program. To compare between c-ELISA and conventional RT-PCR for diagnosing FMDV using Kappa value in IBM SPSS statistics 19 (SPSS Inc.), if Kappa value = 0.81-0.99 it means perfect compatibility between the two tests (Anthony and Joanne, 2005). The relative risk (RR) for the association between risk factors for FMD was verified employing 2 by 2 tables in Epi-InfoTM 7 software (version 7).
RESULTS
In this study, 460 serum samples were tested by c- ELISA technique and 460 saliva swabs were tested by conventional reverse transcription polymerase chain reaction (RT- PCR). The overall prevalence of FMD in Nineveh
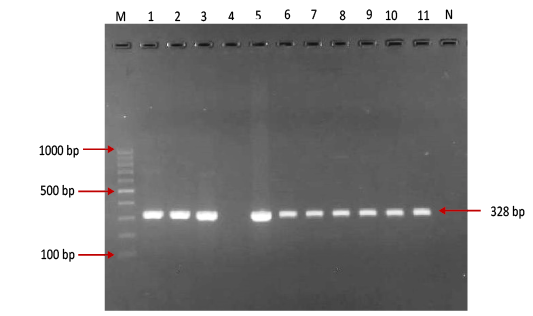
Figure 1: Gel electrophoresis image showing: Lane M) Exact Mark 100 – 1000 bp DNA ladder, Lane 1-11 except 4) conventional PCR technique detected all possible FMDV in approximately band size 328bp. Lane 4) is negative result, Lane N) DNA extracted from FMDV-free cattle used as negative control.
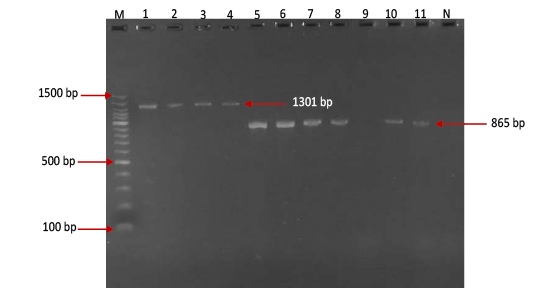
Figure 2: Gel electrophoresis image showing Lane M) Exact Mark 100 – 1500 bp DNA ladder, Lane 1-4) conventional PCR technique detecting genotype O of FMDV in approximately band size 1301 bp. Lane 5-11except 9) conventional PCR technique detecting genotype A of FMDV in approximately band size 865 bp, Lane 9) is negative result, Lane N) DNA extracted from FMDV-free cattle used as negative control.
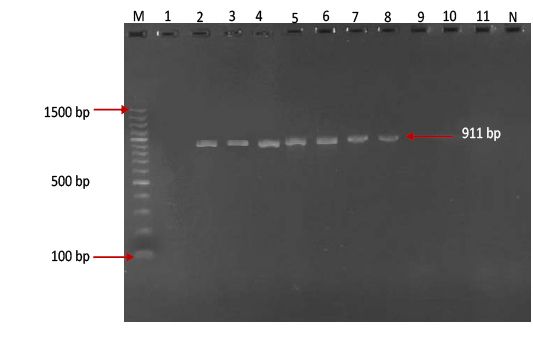
Figure 3:Gel electrophoresis image showing Lane M ) Exact Mark 100 – 1500 bp DNA ladder, Lane 2-8) conventional PCR technique detecting genotype Asia-1 of FMD virus in approximately band size 911 bp. Lane1,9,10,11) are negative results, Lane N) DNA extracted from FMDV-free cattle used as negative control.Gel electrophoresis image showing Lane M ) Exact Mark 100 – 1500 bp DNA ladder, Lane 2-8) conventional PCR technique detecting genotype Asia-1 of FMD virus in approximately band size 911 bp. Lane1,9,10,11) are negative results, Lane N) DNA extracted from FMDV-free cattle used as negative control.
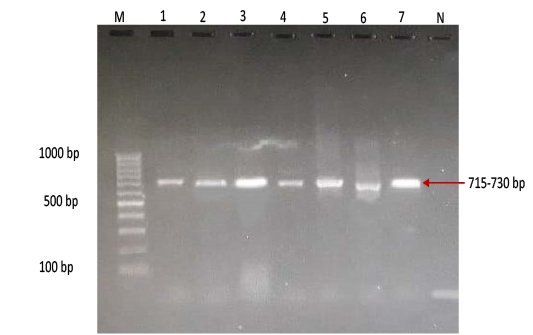
Figure 4: Gel electrophoresis image showing Lane M) Exact Mark 100 – 1000 bp DNA ladder, Lane 1-7) conventional PCR technique detecting only genotype SAT of FMD virus in approximately band size 715- 730 bp. Lane N) DNA extracted from FMDV-free cattle used as negative control.
province was 46.95% (216 out of 460) and 40.43% (185 out of 460) by the two tests used respectively (Table 2). There was perfect compatibility between c- ELISA test and conventional RT- PCR technique, as Kappa value was 0.81 which means that both tests were highly efficient for diagnosis of FMD in cattle (Table 3). The positive bands for all possible FMDV genotypes were at approximately 328bp (Figure 1) with detection rate of 40.43% (Table 4), comprising: A genotype positive bands at approximately 865bp (Figure 2),O genotype positive bands at approximately 1301bp (Figure 2), Asia-1 genotype positive bands at approximately 911bp (Figure 3) and SAT genotype positive bands at approximately 715–730 bp (Figure 4), and genotype C was not detected, with detection rates of 12.39%, 6.95%, 17.39% 3.69% and 0.00% respectively (Table 4).
Table 2: The overall prevalence of FMD infection in cattle employing c-ELISA and RT-PCR technique in Nineveh province.
| Type of test | No. of examined samples |
No. of positive samples |
Percentage of infection (%) |
|
c-ELISA |
460 | 216 |
46.95a |
| RT-PCR | 185 |
40.43a |
Significantly different (P < 0.05) value is labeled with different superscript letters (a or b).
Table 3: Comparison between c-ELISA and conventional RT- PCR technique depending on the Kappa value for diagnosis of FMD.
| RT - PCR | ||||
| Infected | Uninfected | Total No. | ||
|
c- ELISA |
Infected | 183 |
33• |
216 |
| Uninfected |
3•• |
241 | 244 | |
| Total No. | 186 | 274 | 460 | |
•Means false positive. ••Means false negative.
Table 4: Detection rate of FMD genotypes in cattle saliva swabs using conventional RT – PCR technique (n = 460).
| Genotypes | No. of samples tested | No. positive (%) |
| A | 460 | 57(12.39) |
| O | 32(6.95) | |
| Asia-1 | 80 (17.39) | |
| SAT | 17 (3.69) | |
| C |
0 (0.00) |
|
| Overall% | 186(40.43) | |
Table 5: Relative risk of cattle factors associated the seroprevalence of FMD based on c-ELISA test.
| Competitive ELISA test | No. cattle tested | Factors | |||
|
P value |
95%Confidence Interval |
Relative Risk |
No. of positive ( % ) |
||
| Age | |||||
| 1 |
22(29.72)a |
74 | <6 months | ||
| 0.00006 | 1.34 – 2.84 | 1.95 |
86(58.10)c |
148 | >6months-2years |
| 0.017 |
1.04 – 2.22 |
1.52 |
108 (45.37)b |
238 | <2years |
| Gender | |||||
| 1 |
80(33.33)a |
240 | Males | ||
| 0.0001 | 1.50 – 2.28 | 1.85 |
136(61.81)b |
220 | Females |
| Pregnancy | |||||
| 1 |
24(21.81)a |
110 | Non Pregnant | ||
| 0.0000 | 1.649 – 3.59 | 2.434 |
68( 53.12)b |
128 | pregnant |
| Origin | |||||
| 1 |
50(27.77)a |
180 | Native | ||
| 0.0001 | 1.69 – 2.81 | 2.18 |
170(60.71)b |
280 |
Imported |
Values significantly different (P < 0.05) between management factors are labelled with the different superscript letters (a, b or c).
Table 6: Relative risk of the management factors associated the seroprevalence of FMD based on c-ELISA test.
| Factors | No. cattle tested | Competitive ELISA | |||
|
No. of positive ( % ) |
Relative Risk (RR) | Confidence interval 95% (CI) | P value | ||
| Husbandry | |||||
| Outdoor feeding | 87 |
28(32.18)a |
1 | ||
| Indoor feeding | 373 |
188(50.4)b |
1.404 | 1.02-1.92 | 0.019 |
| Herd size | |||||
| Small size ≤ 10 | 110 |
31(28.18)a |
1 | ||
| Large size ≥ 40 | 350 |
185(52.85)b |
1.87 | 1.36-2.56 | 0.0001 |
| Purpose | |||||
| Dairy cattle | 180 |
60(33.33)a |
1 | ||
| Beef cattle | 280 |
156(55.71)b |
1.671 | 1.32-2.10 | 0.0002 |
| Vaccination | |||||
| vaccinated | 210 |
88(41.9)a |
1 | ||
| Non vaccinated | 250 |
128(51.2)b |
1.221 | 1.00-1.49 | 0.046 |
Values significantly different (P < 0.05) between management factors are labelled with the different superscript letters (a, b or c).
Table 7: Relative risk of regional factors associated the seroprevalence of FMD based on c-ELISA test.
| Different parts of Nineveh province | No. cattle tested | Competitive – ELISA | |||
| No. of positive ( % ) | Relative Risk (RR) |
Confidence Interval 95% (CI) |
P value | ||
| Western parts | 31 |
5(16.12)a |
1 | ||
| Southern parts | 60 |
16(26.66)a |
1.65 | 0.66 – 4.09 |
0.25 |
| Northern parts | 117 |
63(53.84)c |
3.33 | 1.47 – 7.58 |
0.0001 |
| Eastern parts | 162 |
93(57.40) d |
3.55 | 1.57 – 8.03 |
0.00002 |
|
Central parts |
90 |
39(43.33)b |
2.68 | 1.16 – 6.30 |
0.006 |
Values significantly different (P < 0.05) between regional factors are labelled with different superscript letters (a, b or c).
Table 8: Relative risk of seasonal factors associated with the seroprevalence of FMD base on c-ELISA test.
| Factors | No. cattle tested | Competitive-ELISA | |||
|
No. of positive ( % ) |
Relative Risk (RR) | 95% Confidence interval (CI) | P value | ||
|
Summer June-July-August |
98 |
28(28.57)a |
1 |
||
|
Autumn September-October-November |
107 |
32(29.9)a |
1.04 |
0.68-1.60 |
0.83 |
|
Winter December-January-February |
85 |
55(64.7)c |
2.26 |
1.59-3.21 |
0.0000009 |
|
Spring March-April-May |
170 |
101(59.41)b |
2.07 |
1.48-2.91 |
0.000001 |
Values significantly different (P < 0.05) between regional factors are labelled with different superscript letters (a, b or c).
Based on c- ELISA test, the seroprevalence of FMD was significantly higher among feedlot calves ( > 6 months – 2years) old 58.10% (RR:1.95 times, CI: 1.34- 2.84) than adult cattle (>2years) and young calves (< 6 months) which were 45.37% , 29.72% respectively. The seroprevalence of infection was significantly higher among females at 61.81% (RR: 1.854 times, CI: 1.508 – 2.280) compared to males at 33.33 %. The seroprevalence of FMD was significantly higher among pregnant animals at 53.12% (RR: 2.43 times, CI: 1.64 – 3.59) compared to non- pregnant animals 21.81%. Moreover, the seroprevalence of FMD showed a significant difference between cattle origin (P<0.05), the imported cattle had a significantly higher prevalence of 60.71% (RR: 2.185 times, CI: 1.695 – 2.816) than the native cattle at 27.77% (Table 5).
The outcomes of the present study showed that the seroprevalence of FMD differed significantly according to the type of animal management, so the seroprevalence of FMD was significantly higher among indoor feeding animals at 50.4% (RR :1.40 times, CI: 1.02 – 1.92) than the outdoor feeding animals at 32.18 %. The seroprevalence was significantly higher among large size of herds (52.85%) (RR: 1.87 times, CI: 1.36 – 2.56) compared to the small size herds 28.18%. The seroprevalence of FMD was significantly higher among beef cattle (55.71 %) (RR: 1.67 times, CI: 1.326 – 2.106) compared to the dairy cattle at 33.33%. In addition, the seroprevalence of the disease was considerably higher among non-vaccinated animals (51.2%) (RR: 1.22 times, CI:1.00 – 1.49) than vaccinated animals (P<0.05) (Table 6).
In general the seroprevalence of FMD was substantially affected by the geographical regions in Nineveh province (P< 0.05). The seroprevalence of FMD was significantly higher in Northern , Eastern and central parts of Nineveh province which were 53.84% , 57.40% , 43.33% respectively with (RR: 3.338 , 3.559 , 2.686 times respectively, CI: 1.470 – 7.580 , 1.577 – 8.030 , 1.163 – 6.302 respectively) compared with the Western and Southern parts of Nineveh province which were16.12%, 26.66% respectively (Table 7).
The seroprevalence of FMD was substantially affected by seasons (P< 0.05). The results revealed that the seroprevalence of foot and mouth disease was considerably higher in Winter and Spring seasons which were 64.70%, 59.41% respectively (RR: 2.264 and 2.079 times respectively) compared to Summer and Autumn seasons which were 28.57%, 29.90% respectively (Table 8).
DISCUSSION
FMD is endemic in Iraq and occurs every year causing highly economic losses, interfering with the mobility of animals and meat between countries and its effect on the international trade (Ali et al., 2018; Dawood and Alsaad, 2018). In this study the overall occurrence of FMD in cattle in Nineveh province was 46.95% and 40.43% based on c-ELISA test and conventional RT-PCR respectively. These results are lower in comparison with earlier studies by: Al-Rodhan, (2014) who stated that the occurrence of FMD in cattle in Basrah governorate was 94.5% and 75.3% using FMD 3ABC Bo Ov ELISA test and conventional RT- PCR respectively. The prevalence of FMD among cattle in Al-Qadisiyah governorate, Iraq was 73.3% using RT- PCR technique (Mansour et al., 2018). The seropositive FMD in cattle in Karbala and Al-Najaf in Iraq was 100%, and 75% respectively (Al-Budeiri, 2012). The prevalence of FMD in calves in Mosul, Iraq was 91.5%, using competitive ELISA test (Abd-Alhameed and Rhaymah 2010a). The seroprevalence of FMD in Middle and South of Iraq was 61.42% using FMD 3ABC Bo Ov ELISA test (Abood et al., 2009). Also, Ahmadi et al. (2013) who indicated that the occurrences of FMD in Iran were 91.3% and 90.7% using antigen ELISA test and conventional RT- PCR technique respectively. The prevalence in cattle in all Iraqi provinces was 68.7% (Al-Salihi, 2019). The results in our study are higher than the overall seroprevalence of the disease in cattle in Al-Diwaniyah and Diyala provinces in Iraq which were 34.09% and 25.33% respectively (AL-Jobori, 2012, Al-Ajeeli et al., 2018). Additionally Hefnawy et al. (2018) mentioned that the prevalence of FMD in Egyptian calves was 31% using RT- PCR technique. These higher or lower prevalences were probably due to the control programs of FMD in their country, nature of animal population, different management systems, intervention and agro-climatic conditions (Belina et al., 2016; Al-Ajeeli et al., 2018).
The current study revealed that both tests used in this study were extremely efficient for diagnosing FMD in cattle, and concurs with the results of Ahmadi et al. (2013) and Al-Rodhan and Salem (2014). For the first time four genotypes of FMDV (A, O, Asia-1 and SAT genotypes) were identified in Nineveh province, Iraq with detection rate of 12.39%, 6.95%, 17.39% and 3.69% respectively. These genotypes had been previously reported in other provinces of Iraq: genotype A was reported in Basrah and Al-Qadisiyah governorates (27.3% and 100%) (Al-Rodhan, 2014; Mansour et al. (2018), genotype O was reported in Middle and South of Iraq (61.42 %) (Abood et al., 2009), genotype Asia-1 was reported in Sulaimani governorate (Baba Sheikh et al., 2017), and genotype SAT-1 was reported in 1962 in all Iraqi provinces (VSI Reports, 2009). The different genotypes reported in this study may be due to the circulation of FMDV in different Iraqi provinces for a very long time which lead to mutations of the virus and continuous import of feedlot animals from different endemic countries which may be carriers for FMDV genotypes (Drake and Holland, 1999; Aktas et al., 2015; OIE, 2015). Furthermore, haphazard vaccination programs used in Iraq.
The current study showed significantly higher seroprevalence of FMD among feedlot calves (6 months – 2 years) in comparison with young calves (< 6 months) and adult cattle (> 2 years), most probably attributable to low efficiency of regulatory vaccination programs. Most of these feedlot calves were imported from different countries in which the disease is endemic, and also attributable to improper management practices such as overcrowding in the herd. In contrast to the results of Kibore et al. (2013) and Al-Ajeeli, et al. (2018); Mesfine et al. (2019) stated that higher seroprevalence of the disease in adult cattle compared to young calves. The reason could be the high frequency of exposure of adult cattle to the virus through animals particularly in pastoral areas in search of water and pasture and intermingling with wildlife animals. However, Belina et al. (2016) and Al-Rodhan, (2014) mentioned that the seroprevalence of bovine FMD was not statistically different among various age groups of the animals.
The seroprevalence of FMD was significantly higher among females than males, and this result is in agreement with Chepkwony et al. (2012) and Al- Ajeely et al. (2018). The higher prevalence of FMD in females might be attributed to the economic benefit more than the males and physiological factors such as estrus, pregnancy and lactation in females (Nawaz et al., 2014; Mansour et al., 2018). On the contrary, higher seropositivity has been reported in males than female animals (Megersa et al., 2009; Mohamoud et al., 2011),while Belina et al. (2016) reported no significant difference in seroprevalence of FMD between male and female animals.
The seroprevalence of FMD in the present work was considerably higher among pregnant cattle than non-pregnant cattle, which may be surprising as pregnant cattle receive better management, but it may be attributed to stress, physiological and hormonal factors associated with pregnancy (Susan, 1998). This outcome is compatible with Rahman et al. (1989) and Fakhrul-Islam et al. (2016) who stated that the pregnant animals were more susceptible to FMD than non-pregnant animals, but incompatible with Al-Ajeeli et al. (2018) who mentioned that there was no significant difference between pregnant and non-pregnant cattle infected with FMD.
The seroprevalence of FMD was considerably higher among imported than native animals, and this might be due to the fact that these animals were imported from countries where the disease had been documented, such as Iran (Ahmadi et al., 2013), Syria (OIE, 2015), and Turkey (Aktas et al., 2015). Furthermore, Ramanon, (2016) mentioned that the imported cattle were about three times more likely to be non structural protein (NSP) positive than the local cattle, attributed to household rearing system of imported cattle that improves the spread of the disease and the rearing of crossed breeds in intensive or semi intensive system may increase the likelihood of disease occurrence, while there is free rearing system for the native cattle which lowers the chances of disease occurrence.
The seroprevalence of FMD in the current study was significantly higher among indoor and large size of cattle herds compared to outdoor and small size herds, These results are in line with earlier findings of Hayama et al. (2013); Muroga et al. (2012); Nishiura et al. (2014). Indoor and large size herds have a high level of FMDV shedding, overcrowded animal-facilities with high contact, and low biosecurity levels which play an important role in spreading the disease in the farms (Alexandersen et al., 2003; Hayama et al., 2019). Furthermore, Phouangsouvanh, (2009) stated that a farm with more than 20 heads of cattle was 1.39 times more likely to have FMD infections in comparison with farms with less than 20 heads of cattle.
In the current study the seroprevalence of FMD was significantly higher among beef cattle than dairy cattle, and this result is incompatible with Ramanon, (2016) who reported that the seroprevalence of FMD in dairy cattle was 30.4% which was 1.3 times more than beef cattle which was 25%. The seroprevalence of FMD was considerably higher among non-vaccinated animals than vaccinated animals which might be due to higher levels of FMD non-structural protein antibodies.
The outcomes suggested that the seroprevalence of FMD was considerably higher in the Northern, Central and Eastern regions of Nineveh province compared to Western, Southern regions. This difference in the seroprevalence between regions may be related to management practices, host activity, sampling size, breeds of the existent cattle, herd sizes, origin of animals and efficiency of regulatory vaccination programs. This finding corresponds with those of other researchers (Abbas et al., 2012; Kibore et al., 2013; Babangida et al., 2017; Mesfine et al., 2019).
The results revealed that the seroprevalence of FMD was considerably higher in winter and spring in comparison with summer and autumn seasons. This finding is compatible with Mannan et al. (2009); Sarker et al. (2011) and Rahman et al. (2015) who verified that the seasons affect the occurrence of FMD.
CONCLUSION
FMD is prevalent among cattle in the Nineveh province. For the first time four genotypes of FMDV (A, O, Asia1 and SAT) were detected in Nineveh province. There are several risk factors that play important roles in the seroprevalence of FMD including feedlot cattle, females, pregnancy, imported, indoor feeding, large herds size, beef cattle, non-vaccinated, location and seasons.
ACKNOWLEDGEMENTS
The authors wish to thank the College of Veterinary Medicine/ University of Mosul for financially supporting the surveillance work, the expert technical assistance from veterinary clinical pathology, laboratory of veterinary teaching hospital, Staff of the department of internal and preventive Medicine for their support and the animal’s owners for their cooperation.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
authors contribution
All authors contributed substantially to this study and are in full agreement with the content of the manuscript.
REFERENCES