Advances in Animal and Veterinary Sciences
Research Article
The Influence of Kebar Grass (Biophytum petersianum K.) Ethanol Extract Towards the Amount of Spermatogenic Cells of Mice (Mus musculus) Induced by 2,3, 7, 8 Tetrachlorine Dibenzo-p-dioxin
Dhanar Adhitya Sukma Fambudi1, Sri Mulyanti2, Dewa Ketut Meles3, Epy Muhamad Luqman4, Widjiati4*
1Faculty of Veterinary Medicine, University of Airlangga, Mulyorejo Surabaya, 60115 Indonesia; 2Department of Reproduction, Faculty of Veterinary Medicine, University of Airlangga, Mulyorejo Surabaya, 60115 Indonesia; 3Department of Basic Veterinary Medicine, University of Airlangga, Mulyorejo Surabaya, 60115 Indonesia; 4Department of Veterinary Anatomy Faculty of Veterinary Medicine, Universitas Airlangga, Mulyorejo Surabaya, 60115 Indonesia.
Abstract | The 2,3,7,8 Tetrachlorine Dibenzo-P-Dioxin (TCDD) were found as product of industrial or agricultural waste could cause abnormalities in the male reproductive organs. Kebar grass were a typical plant in Papua which were rich in Vitamin E as an antioxidant can reduce the effects of free radicals produced by TCDD. The purpose of this study was to determine the effect of ethanol extract of Kebar Grass on the number of spermatogenic cells of male mice induced TCDD. 30 male mice were divided into five groups, each group consisted of 6 replications. Group C(-) was given aquadest, group C(+) was given TCDD 0.14 μg each mice for once. T1 group was given TCDD 0.14 μg each mices for once and given Kebar grass extract 0.045mg/g BW/day. T2 group was given TCDD 0.14 μg each mices for once and given Kebar grass extract 0.080 mg/g BW/day, and T3 group was given TCDD 0,14μg each mices for once and given Kebar Grass extract 0.135mg/g BW/day. All treatment given for 53 days and testes were taken to be made as histopathology preparation to calculate the average number of spermatogenic cells. The results of this study indicate that there were significant differences (p<0.05) between group. But there’s no significant differences (p>0.05) treatment of group average spermatogenia cells, administration of Kebar Grass extract can maintain the number of cells primary spermatocyte and spermatid cell counts. The treatment group with 0.135 mg/g dose showed the best dose to increase primary spermatocyte and spermatid cell counts.
Keywords | Testes, Mus musculus, TCDD, Spermatogonia, Primary spermatocyte, Spermatid
Received | May 10, 2020; Accepted | July 15, 2020; Published | August 04, 2020
*Correspondence | Widjiati, Department of Veterinary Anatomy Faculty of Veterinary Medicine, Universitas Airlangga Jl. Mulyorejo Surabaya, 60115 Indonesia; Email: widjiati@fkh.unair.ac.id
Citation | Fambudi DAS, Mulyanti S, Meles DK, Luqman EM, Widjiati (2020). The influence of kebar grass (Biophytum petersianum K.) ethanol extract towards the amount of spermatogenic cells of mice (Mus musculus) induced by 2, 3, 7, 8 tetrachlorine Dibenzo-p-dioxin. Adv. Anim. Vet. Sci. 8(9): 976-981.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.9.976.981
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Fambudi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
As the population and activities increase, the amount of waste generated by urban areas increases from time to time. Because of the increasing amount of waste, if not managed properly, urban waste will have a negative impact (Wahyono and Setiyono, 2002). The composition of the waste produced by organic waste and the rest is non-organic waste. Most plastic waste is a type of plastic bag or plastic bag in addition to plastic packaging. (Purwaningrum, 2016).
One of the dangerous substances produced from waste is 2, 3, 7, 8-Tetraclorodibenzo-P-Dioxin, the most toxic compound of the dioxin group. Has a long half-life, bioaccumulation in the body, and can be found in fat tissue, blood serum and breast milk (Birnbaum et al., 1994). According to Alsharif et al. (1990) that 2, 3, 7, 8-Tetrachlorodibenzo-P-Dioxin can also cause oxidative stress which results in lipid peroxidase. In addition 2, 3, 7, 8-Tetraclorodibenzo- P-Dioxin is an endocrine disruptor that suppresses every hormone system in the body (Foster et al., 2005).
According to Calivarathan et al. (2002) TCDD is a toxic material to the reproductive system and can reduce fertility, suppress growth and maturation of spermatozoa and reduce levels of testosterone, Gonadotropin Releasing Hormone (GnRH), Follicular Stimulating Hormone (FSH) and Luteinizing Hormone (LH). In addition, TCDD can damage antioxidant defenses, and can reduce reproductive capacity.
Exposure 2, 3, 7, 8 Tetraclorodibenzo-P-Dioxin in male mice can cause decreased survival of spermatozoa (Bell et al., 2007), decreased quality of male reproductive organs (Gray et al., 1997). Resulting in weight loss from the reproductive organs (Bjerke and Peterson, 1994). With the reduction in weight of the reproductive organs, animals will experience adult sex delay (Faqi and Chahoud, 1998). There was also a decrease in the number of spermatozoa due to exposure to certain doses of dioxin (Mably et al., 1992)
Kebar grass (Biophytum petersianum K) or regional language Banondit is one of the plants that grow in the Kebar District of Papua, Indonesia. Banondit has been known for generations by the Kebar community as a fertility drug for both humans and livestock as stated by Unitly and Inara (2011). According to Sembiring and Darwati (2014) Kebar Grass contains several compounds namely alkaloids, saponins, tannins, flavonoids, triterpenoids and glycosides. Some active ingredients from this groupcan be beneficial to health, namely flavonoids, and saponins. Flavonoids that are abundant in the Kebar Grass plant tissue can act as antioxidants (Robinson, 1995).
In addition, the vitamin E content in Kebar Grass is quite high, according to Yin et al. (2012) vitamin E is useful for protecting leydig cells from oxidative stress due to TCDD exposure, so that testosterone hormone production can be maintained, increasing testosterone hormone will increase spermatogenic cell production in seminiferous tubules (Latchoumycandane and Marthur, 2002). Saponins in the Kebar Grass are compounds that are useful for the formation of testosterone, besides the flavonoid compounds in the Kebar Grass also increase the hormone testosterone (Lefaan, 2014).
TCDD has been shown to cause oxidative stress in the testes and has decreased the number of spermatogenic cells (spermatogonia, primary spermatocytes and spermatids). Kebar grass has been shown to contain antioxidant flavonoids and vitamin E, can the administration of Kebar Grass extract have the potential to treat oxidative stress by TCDD? This study aims to determine the potential of Kebar Grass in treating the decline in spermatogenic cells (spermatogonia, primary spermatocytes and spermatids) due to TCDD exposure.
MATERIALS AND METHODS
This experimental laboratory study was carried out for 60 days using a completely randomized design. The instrument used in this study was a test animal cage, minor surgical instruments and a gavage. The research materials used were mice, 2, 3, 7, 8-tetrachlorinedibenzo-p-dioxin solution, 96% ethanol, rat feed, CMC-Na, 10% formalin, 80% alcohol, 90% and 95% for dehydration preparations, xylol, liquid paraffin, haemotoxilin major solution and aquadest.
Animal treatment
After the animal adaptation procedure is completed, a sample randomization is performed. The sample of this study were male mice aged 3 months with the certified ethical clearance. A total of 30 male mice were randomized into 5 groups, each group consisted of 6 replications. The treatment group consisted of 5 groups: C (-): The control group was given 0.1 ml aquadest and 0.5% CMC-Na. C (+): TCDD control group dose 0.14 µg/head once intraperitonially and continued with aquadest 0.1 ml orally once a day. T1: The treatment group was exposed to TCDD 0.14 µg/head intraperitonially and 0.045 mg/gBW of Kebar Grass extract in 0.5% CMC-Na as much as 0.1 ml orally. T2: The treatment group was exposed to 0.14 µg/tail TCDD intraperitonially and 0.080 mg/gBW/day in Kebar Grass extract in 0.5% CMC-Na as much as 0.1 ml orally. T3: The treatment group was exposed to TCDD 0.14 µg / head intraperitonially and 0.135 mg/gBW/day in Kebar Grass extract in 0.5% CMC-Na as much as 0.1 ml orally. All treatment given orally once a day for 53 days and testes were taken to be made as histopathology preparation to calculate the average number of spermatogenic cells. Analysis of the results was using One Way Anova test and calculated using Statistical Program for Social Scientific (SPSS) version 21.
RESULTS AND DISCUSSION
Based on the results of the statistical analysis of the average spermatogonia cells, there were a significant difference between groups (C-) and a group (C+) (p <0.05). The administration of Kebar grass extract in groups T1, T2 and T3 had not had a significant effect (p>0.05). This research showed that the administration of Kebar grass extract could’n improve/increase the number of spermatogonia cells, although there was a tendency to increase the number of spermatogonia with the increase dose of Kebar grass extract administration (Table 1). The average primary spermatocyte cells were a significant difference between groups (C-) and a group (C+) (p <0.05). The administration of Kebar grass extract in the T1 and T2 groups had not had a significant effect on the average of primary spermatocyte cells. The Kebar grass extract could have a significant effect on the T3 group. This research showed that the administration of Kebar grass extract could increase the number of primary spermatocyte cells especially in administering Kebar grass extract at doses 0.135 mg/g BW (T3) (Table 1). The results of a similar statistical analysis were found in spermatid cell counts. The administration of Kebar grass extract had not had a significant effect on the T1 and T2 groups and only has a significant effect on the T3 group on average spermatid cells. This research showed that the administration of Kebar grass extract could increase the number of spermatid cells especially in administering Kebar grass extract at doses 0.135 mg/g BW (T3) (Table 1).
Table 1: Average of spermatogonia cells, primary spermatocytes and spermatids of male mice given TCDD exposed grass extracts.
Treatment |
Average of spermatogonia cell± SD |
Average of Primary Spermatocyte cell ± SD |
Average of Spermatid cell ± SD |
|
C (-) |
87.00b±8.45 |
157.60b±15.43 |
208.00b±26.08 |
|
C (+) |
44.60a±5.77 |
78.40a±6.02 |
121.20a±8.52 |
|
T1 |
70.40ab±6.22 |
96.20a±12.54 |
149.20a±3,11 |
|
T2 |
76.40ab±4.39 |
96.20a±12.54 |
166.80a±10.63 |
|
T3 |
69.00ab±2.73 |
144.00b±5.33 |
192.60b±5.17 |
Superscripts with different letters show significant differences (p<0.05). C (-): Group with aquades and CMCNa 0.5%, C (+): TCDD 7µg/Kg BB, T1: TCDD 7µg/Kg BW + Kebar Grass extract 0.045 mg/g BW/day in a 0.5% CMC-Na solution of 0.1 ml, T2: TCDD 7µg/Kg BW + Kebar Grass extract 0.08 mg/g BW / day in a 0.5% CMC-Na solution of 0.1 ml, T3: TCDD 7µg/Kg BW + Kebar Grass extract 0.135 mg/g BW/day in a 0.1% CMC-Na solution of 0.1 ml
Spermatogenic cells injury (spermatogonia, primary spermatocytes and spermatid cells) as a primary morphologic event is a common manifestation following administration of cytotoxic compounds. Spermatogenic cells not protected by the blood-testis barrier (BTB), are the most vulnerable to toxic effects (Meistrich, 1986). Effects of toxic compounds on the testis may be reversible following cessation of compound exposure through seminiferous epithelial reconstitution (Roeser et al., 1978).
Most of the toxicity of TCDD via the Aryl hydrocarbon Receptor (AhR) receptor, after binding to the AhR, the bonding complex changes and is translocated to the nucleus. Furthermore, it binds to the transcription factor, which is the Ribonucleic Acid Transferase (Arnt) in the nucleus and is involved in translating so that it encodes the cytochrome P450 gene and increases the production of the cytochrome P450 enzyme which results in cell damage (Dobrzyński et al., 2009). Damage to spermatogenic cells results in cells failing to divide, so the number of spermatogenic cells is reduced compared to normal (Gray et al., 1997). TCDD produces high free radicals, which can damage cells through lipid peroxidase. Testicular cell membranes are formed from lipids that are susceptible to free radicals (Alsharif et al., 1990). ROS reaction to unsaturated lipids in the cell membrane causes the formation of lipid peroxide, causing damage to body cells (Yin et al., 2012).
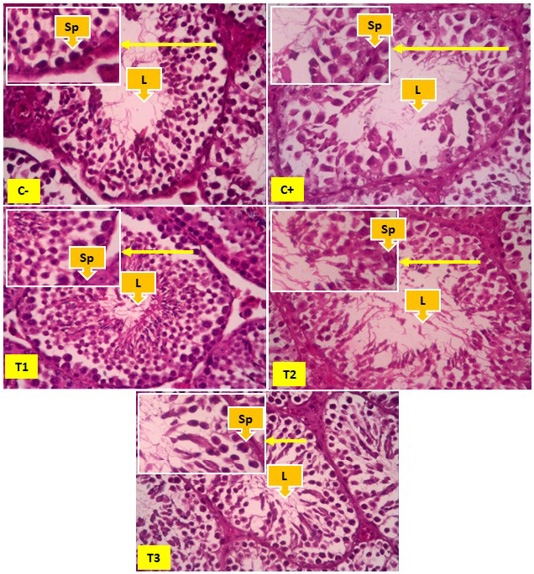
Figure 1: Overview of spermatogonia cells in seminiferous tubules, yellow arrows show spermatogonia cells (Sp), and Lumen (L). In Figure 1 it appears that the number of spermatonia was more common in the negative control group (C-) than the positive control group (C+). The number of spermatogonia appears to be increasing, although not significantly found in the T1, T2 and T3 groups.
The presence of lipid peroxidase in cell membranes due to TCDD free radicals resulted in a decrease in the number of spermatogonia, primary spermatocytes and spermatid cells in the control group exposed to TCDD (C+) compared to the control negative (C-). The administration of Kebar grass extract in groups T1, T2 and T3 had not had a significant effect of the average spermatogonia cells (p>0.05). The administration of Kebar grass extract in the T1 and T2 groups had not had a significant effect on the average of primary spermatocyte and spermatid cell counts. Only the Kebar grass extract could have a significant effect on the T3 group (primary spermatocyte and spermatid cell counts) (Table 1).
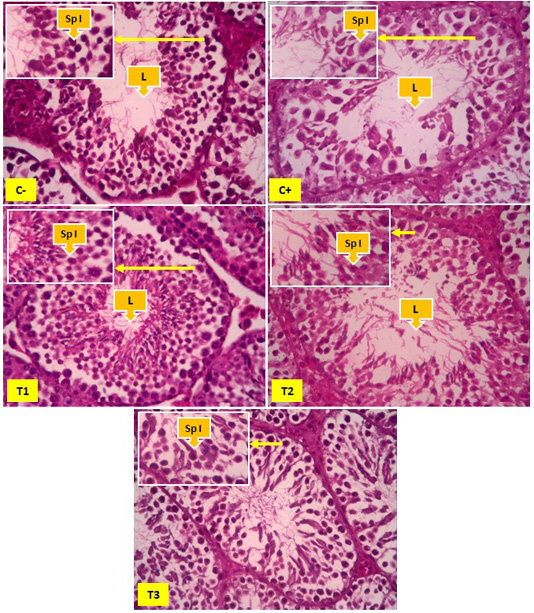
Figure 2: Overview of spermatocyte cells in the seminiferous tubules, yellow arrows indicate primary spermatocyte cells (Sp I) and Lumen (L). In Figure 2 it appears that the number of primary spermatocyte cells was more common in the negative control group (C-) than the positive control group (C+), T1 and T2. The number of primary spermatocyte cells appears to be increasing significantly found in the T3 groups.
Kebar grass extract as a therapy from TCDD exposure contains antioxidants that are used against the effects of TCDD. Kebar grass contains flavonoids and vitamin E which are high enough to be used to ward off ROS from TCDD. The content of Kebar Grass which is high in vitamin E and flavonoids is proven to be able to maintain the spermatogenic number of mice exposed to TCDD according to Leefan (2014) and Latchoumycandane and Marthur (2002). Kebar grass also contains high flavonoids that are useful as antioxidants in TCDD exposure because they have hydroxyl groups that can donate hydrogen atoms to free radical compounds and stabilize ROS (Rezaeizadeh et al., 2011).
Vitamin E were fat soluble so that it easily enters through cell membranes and protects Poly Unsaturated Fatty Acid (PUFA) so that it can protect cells against free radicals by breaking free radicals so that they cannot damage cells (Wati et al., 2014). Vitamin E is also able to convert peroxyl radicals resulting from lipid peroxidase into tocopherol radicals that are less reactive, so that cells are not damaged (Hariyatmi, 2004). Kebar grass contains Calcium which is sufficiently able to repair the influx of Ca and K ions in the testes whose permeability has been damaged by TCDD, thus maintaining spermatogenic cells to remain normal and avoid cell damage (Whitaker, 2006).
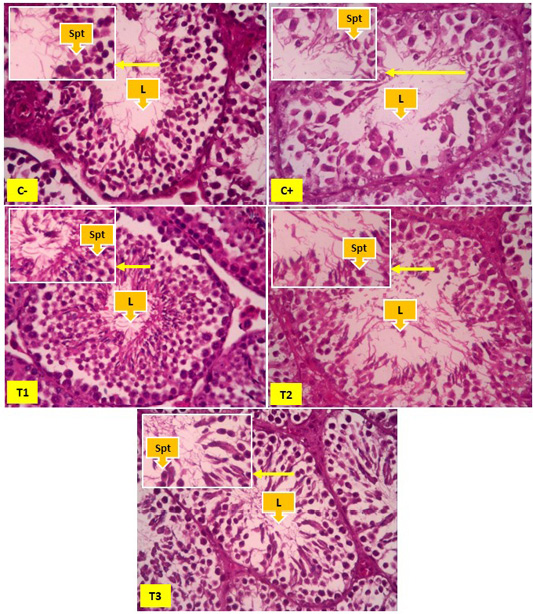
Figure 3: Spermatid cells in seminiferous tubules, yellow arrows showing spermatid cells (Spt) and Lumen (L). In Figure 2 it appears that the number of spermatid cells was more common in the negative control group (C-) than the positive control group (C+), T1 and T2. The number of spermatid cells appears to be increasing significantly found in the T3 groups.
The administration of Kebar grass extract had not had a significant effect on the average of spermatogonia (All treatment), cells primary spermatocyte and spermatid cell counts (T1 and T2), but could have a significant effect on the T3 group (Table 1). The treatment group with 0.135 mg/g BW/day (T1) Kebar Grass dose showed the best dose because it was not significantly different from the control group without TCDD exposure (C-). A similar study was carried out by Rusyawardani (2020) which stated that giving Kebar grass a dose of 0.135 mg/g BW/day gave the best effect in increasing the diameter of seminiferous tubules and the thickness of seminiferous epithelium exposed to TCDD. This might be because the dose of Kebar Grass extract can increase the hormone testosterone, according to Bearden and John (1980) that the number of spermatogenic cells is influenced by the number of cell division that depends on testosterone hormone as a trigger.
CONCLUSION
Based on research that has been done, it can be concluded that the administration of Kebar Grass extract can maintain the number of spermatogenic cells specially cells primary spermatocyte and spermatid cell counts that exposed by 2,3,7,8-tetrachlorodibenzo-p-dioxin. The treatment group with 0.135 mg/g BW/day (T1) Kebar Grass dose showed the best dose to increase spermatocyte and spermatid cell counts.
Acknowledgements
The authors express sincere thanks to the Ministry of Research, Technology and Higher Education of the Republic of Indonesia for funding research and Dean Faculty of Veterinary Medicine for providing all necessary facilities and fund for conducting research work.
Authors Contribution
All authors contributed equally to the manuscript.
Conflict of Interest
Authors declare that they have no conflict of interest.
REFRENCES






