Advances in Animal and Veterinary Sciences
Research Article
Effect of Different Doses from ZnONPS on the Pituitary-Testes Axis Function in Adult Male Rats
Wassan Mhammed Husain1, Jawad Kadhim Araak2 , Orooba Mohammed Saeed Ibrahim2
1Al- Yarmouk University College, University of Baghdad, Iraq; 2College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | In our study, ZnONPs was prepared and characterized for investigation of pituitary-testes axis function in adult male rats. Fifty healthy adult male rats selected, weighted 300 ± 50 gm, randomly divided to five group each group have ten animals. One of them group consider as control group and other four group designed as (G1, G2, G3 and G4) group which receive oral dose of ZnONPs in different concentration (5, 10, 20 and 40 mg/kg- B.W) respectively for 56 day. Blood samples were collected via cardiac puncture for serum collection in (zero time, 28 and 56 day of experiment). The results showed significant (P<0.05) elevation of testosterone and FSH hormones concentration in G3 and G4 after 56 day of experimental period as a compared with zero time and 28 day of experiment, whereas LH and inhibin B hormones concentration showed a significant (P<0.05) decrement in all group at 56 day as a compared with control group and other period time. Histological study of testes and pituitary gland showed different pathological changes on these two gland’s tissue and cells as mild to severe effects especially at dose (20 and 40 mg/kg B.W). Histometric study of seminiferous tubules illustrated a significant (P<0.05) elevation of seminiferous tubules diameters in G4 group, whereas showed non-significant (P˃0.05) differences in number of interstitial cells in treated groups.
Keywords | Zinc oxide nanoparticles, Pituitary testes axis, Testosterone hormone, Oxidative stress, Histometric
Received | January 07, 2019; Accepted | March 12, 2019; Published | May 11, 2019
*Correspondence | Wassan Mhammed Husain, Al- Yarmouk University College, Department of Medical Laboratory Techniques, Baghdad, Iraq; Email: ww88243@gmail.com
Citation | HusainWM, Araak JK, Ibrahim OMS (2019). Effect of different doses from znonps on the pituitary-testes axis function in adult male rats. Adv. Anim. Vet. Sci. 7(7): 550-556.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.550.556
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Hussain et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Nanotechnology term refers to the ability to manipulate measure and organize matter at the nano-scale level. This scale classically indicate to matter in the size range from 1 to 100 nm (Mattsson et al., 2018) also, it is often extended to involve materials below (1 μm) in size (Devalapally et al., 2007).
ZnO is a metal oxide characterized as a semiconducting metal. Zinc oxide nanoparticles (ZnONPs) has drawn interest in past few years ago because its widely range of application in the field of optics, electronics, and biomedical systems (Gunalan et al., 2012; Anbuvannan et al., 2015). Many types of inorganic metal oxides have been created and used in many studies such as CuO, TiO 2, and ZnO. from all these metal oxides, ZnONPs has been interest because they are safe, inexpensive to syntheses, and can be prepared easily (Jayaseelan et al., 2012).
ZnONPs have many properties such as higher catalytic activity, UV filtering properties optic, anti-inflammatory and wound healing (Sheikhloo et al., 2011; Mirzaei and Darroudi 2017). Because its UV filtering properties, ZnONPs has been widely used in cosmetics such as sunscreen lotions (Wodka et al., 2010; Seo et al., 2018). ZnONPs was used in biomedical applications such as anticancer, drug delivery, antibacterial, antidiabetic, antifungal and agricultural properties (Hassani et al., 2015). Although ZnO is used for delivery of drug, it still has the cytotoxicity (Ma et al., 2013). As a result of the extensive uses and much application of ZnONPs among various industries, it is necessary to investigate the effects of ZnONPs on the biological systems, mainly on the male reproductive system, for that pourposis the study was design.
Materials and methods
Preparation and Characterization of ZnONPs
Pomegranate (Punicagranatum) peel extract and (0.1 M) of zinc nitrate crystal [Zn (NO3)2.6H2O] are used in preparation of ZnONPs (Geetha et al., 2016). Physical characterization of nanoparticles by Scanning electron microscopy (SEM) (Ishwarya et al., 2018), X-ray diffraction (XRD) (Bunaciu et al., 2015) and Fourier transform infrared (FT-IR) spectroscopic (Chan and Kazarian, 2016). These tests was report the purity, hexagonal shape and size rang (20-50 nm) of ZnONPs was obtained.
Experimental Animals Design
Fifty adult male rats aged 3 - 3.5 month, weighted 300 ± 50 gm. Rats obtained from animal house of Veterinary Medicine College for Baghdad University. They were housed five per cage, and were placed in room for two weeks for acclimation. Room temperature was maintained at (21 - 25˚c), air of the room was changed continuously by using ventilation vacuum and with light /dark cycle of 12:12 h/day. The litter of the cages was changed every 2 day. Animals were fed on pellet of freshly prepared ration. The experiment animals were randomized design and treated as following:
Fifty adult male rats divided randomly into five groups (ten animals per group). Control group,ten adult male rats treated orally with 2 ml distal water daily via Gavage needle for 56 day .T1 group, ten adult male rat treated orally with (5 mg/kg body weight) of ZnONPs daily via Gavage needle for 56 day. T2 group, ten adult male rat treated orally with (10 mg/kg body weight) of ZnONPs daily via Gavage needle for 56 day.T3 group, ten adult male rat treated orally with (20 mg/kg body weight) of ZnONPs daily via Gavage needle for 56 day.T4 group, ten adult male rat treated orally with (40 mg/kg body weight) of ZnONPs daily via Gavage needle for 56 day, doses was calculated according to (Reza et al., 2014)
Specimen Preparation
Animals were anesthetized by I.M injection of (90mg/kg) Ketamine and (40 mg/kg) Xylazine. Blood samples were collected at (Zero, 28, 56) days of experiment via cardiac puncture technique. Serum separated from coagulated blood sample by centrifugation at 2500 rpm for 15 min and kept it by freezing at -20 ̊ C until used (David, 2005). Testosterone hormone, follicular stimulating hormone (FSH), luteinizing hormone (LH) and inhibin B concentration were assessment by using ELISA kit according to Kit manufacturer’s instructions. After animals were killed the histological changes of right testes and pituitary gland was studied.
Statistical Analysis
The Statistical Analysis System- SAS (2012) program was used to effect of difference factors in study parameters. Least significant difference –LSD test and Duncan Multiple Range (ANOVA) was used to significant compare between meansin this study.
Results
Serum Hormones Parameters
Testosterone hormone concentration (ng\ml): The concentration of testosterone hormone was showed in Figure (1). The result register that non-significant (P ˃ 0.05) difference in testosterone concentration in all treated group as a compared with control group at zero time. While, the result elucidated that was a significant (P ˂ 0.05) elevation of testosterone concentration in G2, G3 and G4 group as a compared with control and G1 treated group at 28 day of treatment. Also showed significant (P ˂ 0.05) elevation of testosterone concentration in G4 treated group as a compared with control and G1 and G2 group at 56 day of experiment period. Whereas, G3 group was showed significant (P˂ 0.05) elevation of testosterone concentration when a compared with control and G1 group at 56 day of administration.
Figure (2) refer to the testosterone concentration among the time of administration, the result was showed a significant (P ˂ 0.05) increment of testosterone concentration in all treated group at 56 day of experiment period when compared with zero time of treated period. While the data was showed a significant (P ˂ 0.05) increment of testosterone concentration in G3 treated group at 28 day of administration as a compared with zero time.
Luteinizing hormone concentration (mlU\ml): The mean values of luteinizing hormone concentration (mlU\ml) in adult male rats treated with serial dose of ZnONPs (5, 10, 20 and 40 mg/kg B.W) synthesized from PPE are clarified in figure (3). Non-significant (P > 0.05) differences in serum concentration of LH are observed in all treated groups at zero time and 28 day of experiment period as a compared with control group. Whereas there was a significant (P< 0.05) decrement in serum LH concentration was registered in all treated group as a compared with control group at 56 day of ZnONPs administration period.
Furthermore, the data in Figure (4) showed a significant (P< 0.05) decrease in serum LH concentration in all treated group at 56 day of experiment period when a compared with zero time and 28 day of experiment period.
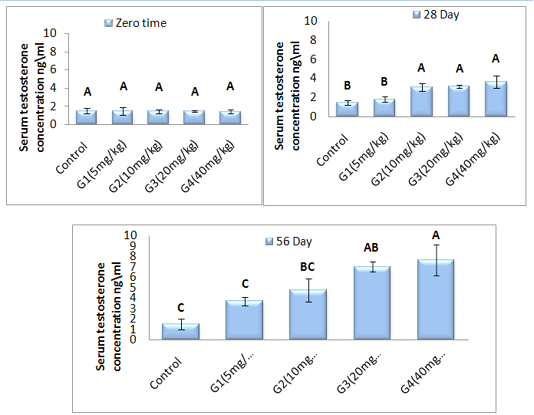
Figure 1: Represent the effect of different doses of ZnONPs was synthesized by PPE on serum testosterone concentration (ng\ml ) in adult male rats along 56 day of treatment.
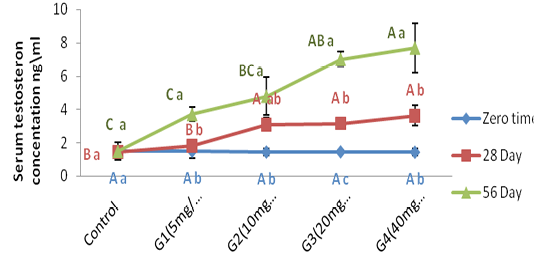
Figure 2: Represent the effect of different doses of ZnONPs was synthesized by PPE on serum testosterone concentration (ng\ml ) in adult male rats in zero time, 28day and 56 day of treatment.
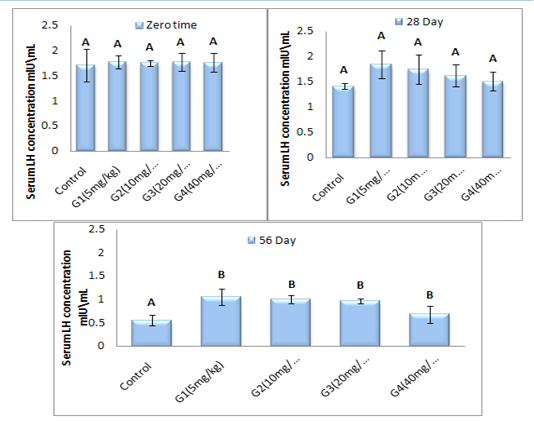
Figure 3: Represent the effect of different doses of ZnONPs was synthesized by PPE on serum LH concentration (mlU\ml) in adult male rats after 56 day of treatment.
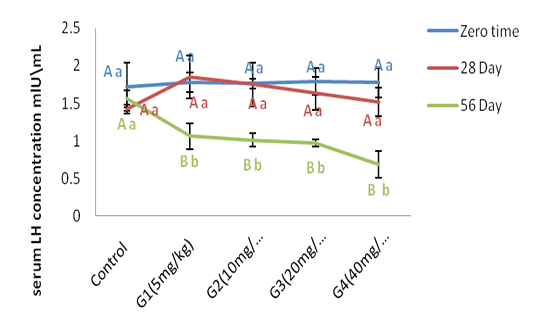
Figure 4: Represent the effect of different dose of ZnONPs was synthesized by PPE on LH concentration (mlU\ml) in adult male rats, in zero time, 28day and 56 day of treatment.
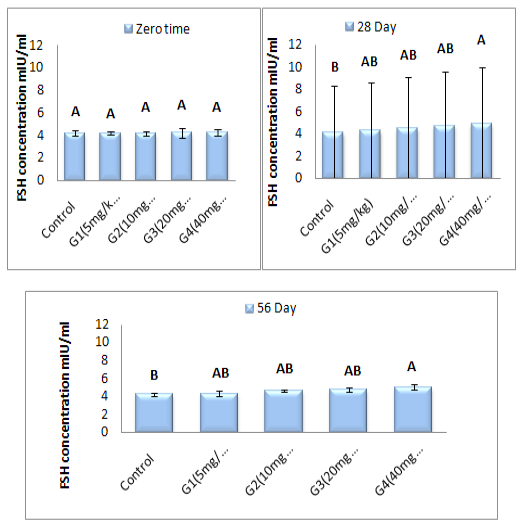
Figure 5: Represent the effect of different doses of ZnONPs was synthesized by PPE on serum FSH concentration (mlU\ml) in adult male rats after 56 day of treatment.
Serum FSH concentration (mlU\ml): Figure (5) refer to the results of FSH concentration (mlU\ml) before and after treated with of ZnONPs synthesized from PPE in adult male rats. FSH concentration at zero time is showed non-significant (P ˃ 0.05) differences in all treated group when compared with control group. Serum follicular stimulating hormone concentration showed a significant (P < 0.05) elevation in G4 treated group at (28 and 56 day) of ZnONPs administration when a compared with control group, while there was non-significant (P ˃ 0.05) differences between G1, G2 and G3 as a compared with control group at the same two periods. From our date in histogram Figure (6) demonstrate significant (P < 0.05) elevation in FSH concentration in G4 group at 56 day of experiment as a compared with zero and 28 of experiment. Between the same group, the concentration of serum FSH was showed in histogram Figure (6). Group treated with 40 mg/kg B.W (G4) of ZnONPs was registered a significant (P < 0.05) increment at 56 day as a compared with zero time and (28 day) in same treated group. Whereas, there were non-significant (P ˃ 0.05) differences in serum FSH concentration in other treated groups at (28 day and 56 day) as a compared with zero time of experiment period.
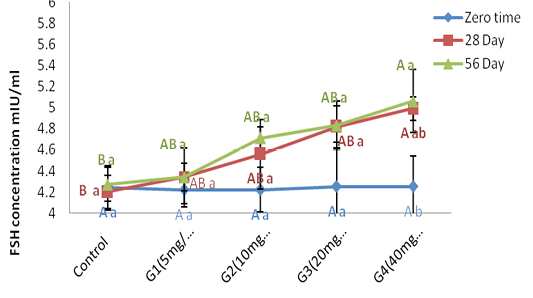
Figure 6: Represent the effect of different doses of ZnONPs was synthesized by PPE on Serum FSH concentration (mlU\ml) in adult male rats in zero time, 28day and 56 day of treatment.
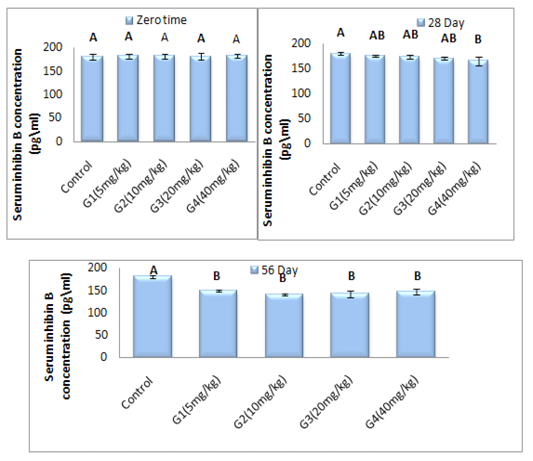
Figure 7: Represent the different doses of ZnONPs was synthesized by PPE on Inhibin B concentration (pg\ml) in adult male rats after 56 day of treatment
Inhibin_B hormone concentration (pg\ml): In this study, the data revealed the effect of zinc oxide nanoparticles in variance doses on the serum concentration of Inhibin B hormone (Figure 7). The results of healthy animals treated with ZnONPs showed significant (P < 0.05) decrease in serum concentration of Inhibin B hormone in G4 treated group at 28 day of experiment period as a compared with control group in same time of treatment. On the other hand, the serum concentration of Inhibin B hormone is registered significant (P < 0.05) decrement in all treated groups with all different dose of ZnONPs at day 56 of administration as a compared with control group. Within time the Inhibin B hormone concentration illustrated in Figure (8) was showed significant (P < 0.05) decrement in all treated group at 56 day of experiment period when a compared with zero time and 28 day of administration accept G4 treated group are non-significant (P>0.05) differences in Inhibin B concentration at 56 day when a compared with 28 day of treatment.
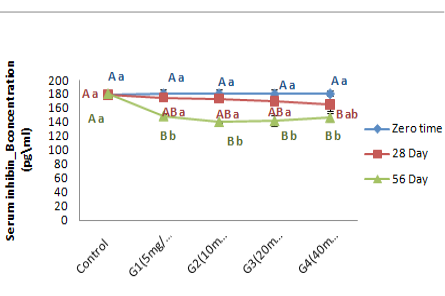
Figure 8: Represent the different doses of ZnONPswas synthesized by PPE on serum Inhibin B concentration (pg\ml) in adult male rats in zero time, 28day and 56 day of treatment.
Histological Study
Histometric study of testicular tissue: The study result showed in Figure (9a, and b) referred to seminiferous tubules diameters (µm) and number of interstitial cells in healthy control animals and healthy animals treated with different doses of ZnONPs for 56 day intervals. The result illustrated a significant (P<0.05) elevation of seminiferous tubules diameters in G4 group as a compared with control group and other groups, whereas, we showed non-significant (P ˃ 0.05) differences in number of interstitial cells in all group when compared with control group at the end of experiment intervals.
Histological changes of pituitary gland: Microscopical examination of healthy control rat’s pituitary gland showed normal view (Figure 10a). Whereas, pituitary gland in healthy rats was received deferent doses of ZnONPs (Figure 10 b,c,d and e) they showed massive swollen of pituitary cells with non- cytoplasmic staining, some of them contain vacuoles and other was atrophy, these vacuoles increase with doses increase. Severe atrophy of acidophil and other cells are necrotic, the basophilic cells are not recognized also the capillary sinusoids dilated and congested. The acidophilic cells become flattened with elongated nuclei and few small capillaries still congested and dilated However in pituitary gland tissue of G4 group.
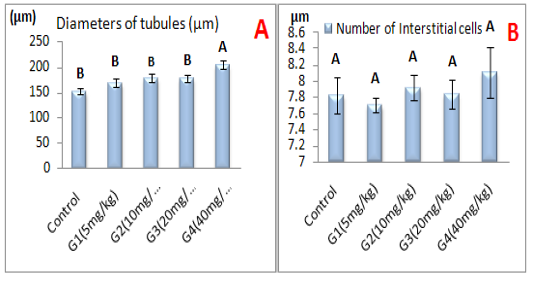
Figure 9: Represent the effect of different doses of ZnONPs was synthesized by PPE on A-Seminiferous tubules diameters (µm), B- Number of interstitial cells.
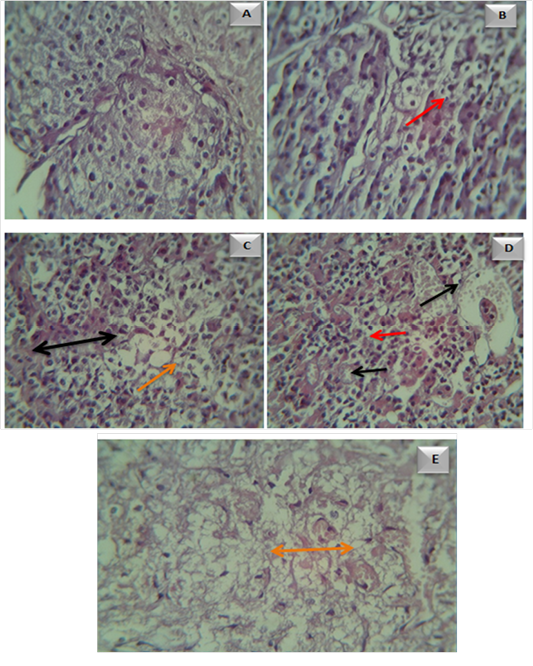
Figure 10: Microscopical examination of pituitary gland tissue in A- control group. B- Adult male rats in (G1 ) group, showed vacuolar formation ( ). C- Adult male rats in (G2 ) group, showed blood vessel congestion and dilation (
). C- Adult male rats in (G2 ) group, showed blood vessel congestion and dilation (  ) with vacuoles (
) with vacuoles ( ). D- Adult male rats in (G3 ) group, showed necrosis(
). D- Adult male rats in (G3 ) group, showed necrosis( ). Adult male rats in (G4 ) group, showed massive vacuolar formation (
). Adult male rats in (G4 ) group, showed massive vacuolar formation ( ).
).
Histological changes in testes: Sections of testicular tissue in adult male rats of control group was showed markedly normal arrangement of the germinal cells and normal architecture Figure (11-a ). Figure (11-b, c,d and e) represent histological effect ZnONPs testicular tissue in G1,G2,G3 and G4 groups respectively. This sections was showed subcapsular congestion, largest and proliferate leydge cells, present of inflammatory cells , thickened and edematous intertubular septa, atrophic necrosis of spermatogonal cells, the remnant cells appeared small. However, most of the seminiferous tubule showed severe atrophic necrosis which appeared elongated and distorted; sometimes appeared small in size when a compared with control.
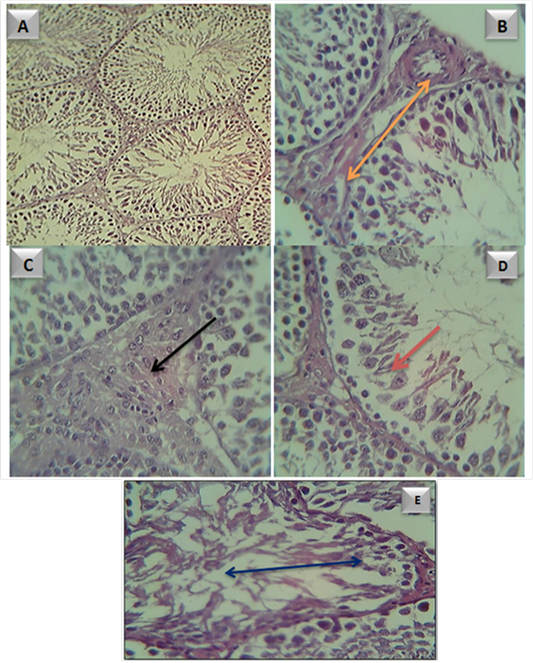
Figure 11: Testicular tissue in A- control group. B- adult male rats in (G1) group, showed subcapsular congestion
( ). C- adult male rats in (G2) group, showed large and proliferate leydge cells (
). C- adult male rats in (G2) group, showed large and proliferate leydge cells ( ). D- adult male rats in (G3) group, showed sever atrophic necrosis of spermatogonal cells (
). D- adult male rats in (G3) group, showed sever atrophic necrosis of spermatogonal cells ( ) E- adult male rats in (G4) group, showed sever atrophic necrosis and distoration of seminiferous tubule (
) E- adult male rats in (G4) group, showed sever atrophic necrosis and distoration of seminiferous tubule ( )
)
Discussion
That elevation of testosteron hormone may result from elevation of cholesterol concentration in blood after treated with ZnONPs (Reza et al., 2014) cholesterol act as precursor molecule for steroid synthesis (Stephenson et al., 2010) play role in increment of steroid hormone in blood after ZnONPs adminstration. Some resercher showed the nanoparticles can be importnt for protein expression (steroidogenic-acut regulatory (StAR)), this protein work as transporter it transferred of cholesterol molecule to the mitochondrial membrane and increase steroid synthesis (Reza et al., 2014). Bara and Kaul. (2018) was observed after exposed of Leydig cells (TM3) from mouse in vitro model to ZnONPs a manifold increment in the relation expression of genes (StAR and P450scc). Profiles of gene expression clearly point to that ZnONPs were having a steroidognesis effect, however, it depend on concentration and the time of exposure to ZnONPs. The elevation of testosteron hormon concentration effect with GnRH secretion from the hypothalamase, which effect on the pituitary gland production of LH, thus may be correlated to easily penetration of ZnONPs from the blood brain barrier (BBB) and blood testicular barrier (BTB) they perhaps its effect on concentration of LH level. The decrement of LH concentration may be result from negative feedback mechanism of hypothalamic-pituitary axis (Badkoobeh et al., 2013).
After 56 day also showed elevation of FSH, this result pointed to effect of doses and time of adminstration on pitutary gland and testicular tissue. Our result aggrement with Shirvani et al. (2014) was showed increment of FSH concentration after receved (25 and 50 mg/kg) ZnONPs for (15 day). Inhibin-B is non-steroidal (glycoprotein) hormone produce from sertoli cell in male gonads (Meachem and Nieschlag, 2001). Inhibin-B act as FSH-regulating in both genders (male and female). By down-regulating effect on FSH production, inhibin-B hormone successfully control on sperm formation and folliculogenesis. Other possible resone, the pathological effect of ZnONPs on liver mayeffect on protein synytesis resulting in decremint of glycoprotein hormne secretion (Fong et al., 2016).
Regardless of the many advantages of NPs, they may produce dangerous effects via special properties including (small size with high surface area) (Attia et al., 2018). Our results demonstrated the miled increment of leydig cell numbers of in animal recived (40 mg/kg) of ZnONPs but it is non significantly difrences when a compared with control group. These result was agreement with our histological study of testes figure ()
Our study were regesterd the histopathological changes in testicular tissue and pitutary gland in rats recived different doses of ZnONPs when a compared with control rats. We were showed these histological changes appear as dose dependent manner, demonstrated the miled increment of leydig cell numbers of in animal recived (40 mg/kg) of ZnONps but it is non significantly difrences when a compared with control group while, ZnONPs was affected sertoli cell via formation of vacuole and immature germ cells sloughed from the seminiferous tubules (Talebi et al., 2013). There is sign demonstrated in a direct sertoli cells damage, and considered as the primary morphological marked of testicular injury and is the highest response of sertoli cells to several xenobiotic (Boekelheide et al., 2000). The occurrence of vacuoles in the sertoli cells cytoplasm was point to direct harm of nanomaterial on these cells (Shirvani et al., 2014), several seminiferous tubules had necrotic sloughed germinal epithelium with modest interstitial edema characterized by present of inflammatory cells , these finding resulted from promotion of oxidative stress (OS) in testicular tissue (Ibrahim, Oda and Khafaga, 2017). Our results was confirmed with Mozaffari et al. (2015) and Bara et al. (2018) they observed that gross histopathological alterations in the testis of prenatally exposed male mice to the ZnONPs. Also, generation of lipid peroxides, change enzymatic activity and finally they induce damage or necrosis (Weber et al., 2003) in the pituitary cells. ZnONP is attributed generally to the production of reactive oxygen species, genotoxicity, inflammation and finally cell death. ZnONPs after oral ingestion can reach to the brain either by blood brain barrier (BBB) breaking or via neural transportation (Shim et al., 2014).
Conclusion
Oral administration of ZnONPs in different doses (5, 10, 20 and 40 mg/kg/ for 56 day intervals have the ability to altered in function of pituitary – testes axis, induce many deleterious effects in pituitary and testes function such as hormonal imbalance, increase oxidative stress in testicular tissue and histopathological changes in pituitary and testes tissue.
Acknowledgements
I like to thank everyone who helped me in this study, especially all staff of Physiology, Biochemistry and Pharmacology department in Veterinary Medicine College in Baghdad University.
Conflict of Interest
No conflict of interest.
Authors Contribution
The authors contributed equally.
References






