Advances in Animal and Veterinary Sciences
Research Article
Application of Differential Interference Contrast Microscope in Studying of Camelus dromedaries Skin
Ghasaq Sami Mshary, Zainab Yahia Kadhim, Karima A. Al Salihi
College of Veterinary Medicine, AL- Muthanna University, Iraq.
Abstract | A significant species differences exist between the skins of the animals according to their lifestyle. The camel is clearly adapted for a desert, and its skin differs in the arrangement and morphology of the hair follicles from that of other domestic mammals. Differential interference contrast microscope (DICM) is related optics give a specimen a three dimensional appearance that enhance depth of focus so that thicker specimens can be observed at higher magnifications.Consequently, this study was designed to dissect the microanatomy of camelid skin and to investigate the arrangement of its layers and structure with special emphasis on sweat glands by validating a differential interference contrast microscope. Skin sample were collected from different regions of five animals (Camelus dromedaries) that slaughtered at Al-Najaf abattoir/Republic of Iraq. The samples were kept in 10% neutral buffered formalin and processed routinely for histopathological sectioning and examined under DICM. The results of this study revealed clear structures of all layers and its cells of the camelids skin. Moreover, DICM revealed the distribution and structure of sweat and sebaceous glands together with the organization of hairs and their follicles that vary from other mammals. In conclusion, this study approved the validity of DICM in the examination of camelids skin. The authors recommend to apply this tool for the examination of normal skin sections as well as validate this technique in diagnosis of skin diseases.
Keywords | Camelids, DICM, Skin, Sweat glands, Sebaceous glands
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 12, 2018; Accepted | June 27, 2018; Published | July 25, 2018
*Correspondence | Ghasaq Sami Mshary, College of Veterinary Medicine, Al- Muthanna University, Iraq; Email: ghassaq51@gmail.com
Citation | Mshary GS, Kadhim ZY, Al-Salihi KA (2018). Application of differential interference contrast microscope in studying of camelus dromedaries skin. Adv. Anim. Vet. Sci. 6(7): 281-285.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.7.281.285
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Mshary et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A significant species difference is reported between the skins of the animals. The desert animals exposed to high levels of solar radiation and high temperatures, therefore, its skin structures have possessed a special adaption to prevent damage of the tissue proteins. The camel is clearly adapted for a desert lifestyle; its skin differs in the arrangement and morphology of the hair follicles from that of other domestic mammals (Fowller, 1998; Bhakat & Sahani, 2005).The skin of camel is unique among domestic animals (Fowller, 1998). It has similar epidermis and dermis layers to that of other hairy mammals. But, it differs in the arrangement of the hairs and the morphology of the hair follicles from that of other domestic mammals (Bhakat & Sahani, 2005). In addition, the distribution and morphology of sebaceous glands is similar to other animals, however, each branch of the compound follicles has their individual ring of sebaceous glands (Taha, 1988). Information about the microanatomy of the skin has been the subject of only few studies in camel (Donald et al., 1962; Quasem et al., 1992; Mahdi, 1979). Previously, researcher stated that the skin of the camel has no sweat glands (Leonard, 1894). However, the scientists approved that sweat glands are present in the camel skin (Leese, 1927; Curasson, 1942; Droandi, 1936; Lee and Schmidt-Nielsen, 1962). Sweat glands has been found to be distributed over the general body surface, one in association with each and cover hair. A special manner attachment is occurred between epidermis and dermis layers in the camel (Simon, 1951). In addition, differences are found in the structure and distribution of skin glands and the arrangement of hairs and their follicles in some respects from that described for other domestic mammals. Camel skin sweat glands were seen to be simple, coiled, tubular glands that appeared over all body areas except of the upper lip, external nares and perianal region (Dowling and Nay, 1962; Gbolagunte, 1983). The rigidity and hardness of skin of the camel is one of the obstacles that make the difficulties in reaching thin histological sections. The thickness of most specimens prevents all parts from coming into focus all at once and limiting the usefulness of higher magnification lenses (Al Salihi et al., 2014).
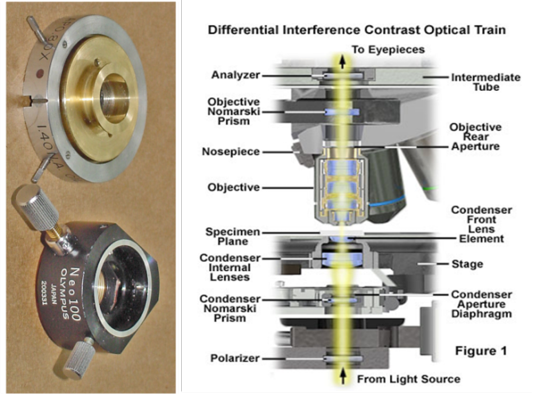
Figure 1: Shows the DIC optical pathSection of intestine showing sloughing of villli and heterophillic infiltration)
The application of light microscope is an essential to perceiving the detail structures of the skin. Bright field microscope is one commonly utilized technique, but it is unable to recognise the more details of skin layers especially in skin of the camel. Differential interference Contrast microscope give a specimen a three dimensional appearance that is not unlike the appearance of a specimen in a scanning electron microscope. These methods enhance depth of focus so that thicker specimens can be observed at higher magnifications (Nomarski, 1955; Francon, 1961). DIC microscopy utilizes the interference between two polarized beams of light that pass through slightly different areas (amount of the shear: D) of a specimen. It visualizes the optical path difference between the beams of light as a differential. DICM requires several optical components; therefore it can be very expensive to set up. Light from an incandescent source is passed through a polarizer, so that all of the light getting through must vibrate in a single plane. The beam is then passed through a prism separates it into components that are separated by a very small distance - equal to the resolution of the objective lens (Figure 1). The beams pass through the condenser, then the specimen. To date, most previous studies on camelid’s skin have been based on conventional light and transmission electron microscopy. As a result, comprehension of three-dimensional (3D) relationship between structures is difficult. A differential interference contrast (DIC) microscopy is a potent tool for obtaining 3D images without any preparation of the specimen (Khalaf and Bainbridge, 1981; Sciorra and Eckert, 1974). Review of literature revealed no publications concerning application of DICM in examination skin of the camel. Consequently, this study designed to use DICM technique in studying normal microstructure of the skin of the camel with emphasising on sweat glands.
Materials and Methods
Skin sample were collected from different regions of five animals (Camelus dromedaries) that slaughtered at Al-Najaf abattoir/Republic of Iraq. The samples were kept in 10% neutral buffered formalin and processed routinely for histological sectioning. Later on, tissues were embedded in paraffin, sectioned into 5-6 µm thickness, and stained with H&E. Slides were examined under DICM and images captured with digital camera connected to microscope (Leica/ Germany) .
Results
Examining normal skin sections of the Camel under DICM revealed a clear epidermis and dermis layers with all their structures and its cells (Figure 2). Under DICM, the epidermis appeared to comprises of keratinized stratified squamous epithelium involves of several sublayers (from most superficial layer to the deepest), these are stratum corneum, stratum lucidum , stratum granulosum, stratum spinosum and stratum basale (germinativum) (Figure 3). The Stratum corneum (horny layer) appeared as the outermost layer and primarily consisted of dying and dead skin cell filled with mature keratin. This layer appeared to occupy ½ -3/4 of the total epidermal thickness and showed a fully keratinized cells pushed up from basal layers. The Stratum lucidum appeared as a dense eosinophilic layer beneath and very close to the stratum corneum. Stratum granulosum (granular layer) appeared as a single layer of cells in some areas and discontinuous in others. Its nuclei was pycnotic, almost of the cytoplasm has been replaced with keratin; Stratum spinosum (prickle layer) was reduced in thickness but revealed daughter cells of the basal layer and was 1-3 cells thick. These cells appeared as a viable and nucleated and actively synthesize keratin. Stratum germinatium (stratum basale) appeared as the deepest layer of cuboidal or columnar cells, most of which was keratinocytes with a few melanocytes.
The dermis appeared as thick inner compartment of the skin. Variations were recognized in the thickness between different parts of the body skin and revealed a superficial layer composed of loose connective tissue interdigitating with undulations in the epidermis and deep dermis, which was composed of connective tissue rich in elastic and collagen fibers in its subepithelial layer, while reticular fiber predominate deep in the compartment. Moreover, an area
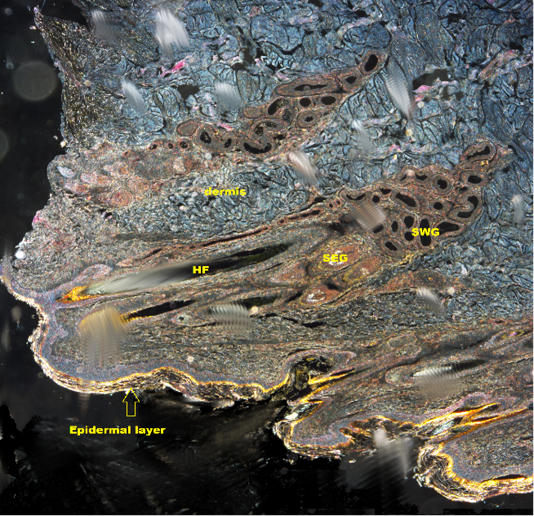
Figure 2: Shows DICM image of the skin of the camel revealing all layers of the skin (X10). HF: Hair follicle; SWG:Sweat glands; SEG: Sebaceous gland
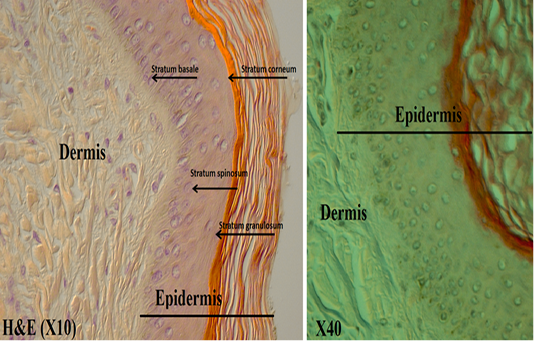
Figure 3: Shows the epidermis layers under DICM
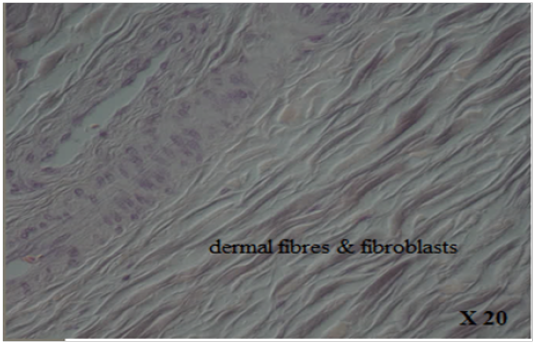
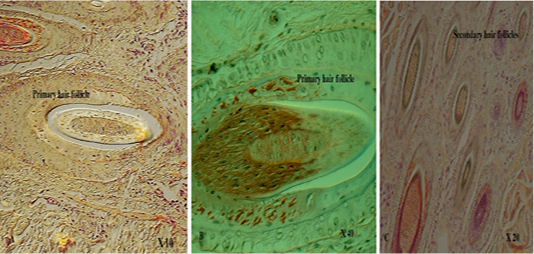
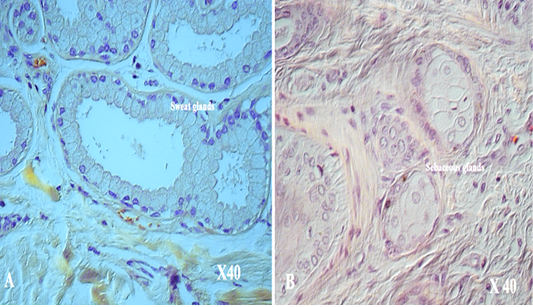
with thick fibers (stratum reticular) was seen. The dermis contains hair follicles, blood and lymph vessels, nerves, and sebaceous and sweat glands. The mid-dermis is characterized by a proliferation of blood vessels, in contrast to that in other domestic animals (Figure 4). Vessel walls are hyalinized. The dermal fibres, its fibroblasts and the dermal ground substance were very clear in all examined sections (Figure 5). Myoepithelial cells surrounding the glands are also seen in the dermis.Hair follicles were observed to be in various stages (anagen, catagen, telogen). The majority of hair shafts showed a small discontinuous medulla. Most primary hairs, especially the giant guard hairs, contain a prominent eosinophilic medulla, while the secondary hairs have variable modulation (Figure 6). The sweat glands were found in all examined sections from the body areas. The glands were always associated with the large cover hair, clustered around the hair bulb in the reticular layer of the dermis with variation in the secretory tubules . The secretory tubules are lined with tall columnar, cuboidal or flat
epithelium that differs greatly in size (Figure 7). The sebaceous glands appeared as simple and branched alveolar and associated with hair follicles. The glands consist of cuboidal cells form sebum and their ducts lined by stratified squamous epithelium.
Discussion
Differential interference microscope also called Nomarski microscope gets benefits of alterations in the light bending by diverse parts of transparent specimens and living cells and enable them to become visible during microscopic evaluation (Francon, 1961; Inoue and Spring, 1997; Inoue 1994). DIC microscope is a light microscope that is used widely to investigate details of structures, organelles and motion in unstained living cells (Murphy, 2002). Moreover, a DIC microscope own high sensitivity in determining sample information and offer horizontal resolution. It is a very attractive microscope for medical and biological research, due to it easiness in use and emergency of commercially available microscope. In the current study, DICM was used in the investigation the microanatomy of the Camelus dromedaries skin. The results of the current study appeared as a good visualization tool for both epidermis and dermis layers of the examined thick skin sections of the camels. Furthermore, DICM was also distinguished between different epidermis sublayers. It was also exposed the dermis structures such as connective tissue, deep various dermal fibres, blood and lymph vessels and hair follicles that appeared in various stage, in addition to fine details structures of sweat and sebaceous glands. Albeit, light microscope is used widely in biological sciences especially for histological investigation, disadvantages occurred such as the blurring image due to the thickness and toughness of camelids skin.DICM used in this study showed it’s succeed to overcome light microscope disadvantages and clear images were captured from examination of skin of the camel. For the authors knowledge this is the first study that used DICM in examination of normal skin of the camels. In conclusion, the results of the current study approved the capturing of impressive high resolution and high contrast images for skin of the camel by using DICM. It also approved the successfulness in identifying the different layers of thick sections of camelids skin and its sublayers and structures. The authors approved that DICM is a technique that deserves broader applications in life sciences. This technique has to be explore from researchers especially in studying the normal histological structures and most modern research level microscope, could be updated to carry out DIC observation, in many cases with just adding of DIC prisms and sliders.
Acknowledgements
The authors would like to thank Assistant professor Dr. Abdoalmir AbdHatem , College of Veterinary Medicine, University of Kufa for collecting the skin of the camels from Al Najaf abattoir.
Conflict of Interest
There is no conflict of interest from any third party for publish this study.
Authors Contribution
All authors contributed equally.
References