Advances in Animal and Veterinary Sciences
Research Article
Assessment of Ameliorative Effects of Zingiber Officinale and Nigella sativa on Streptozotocin-induced Diabetic Rats
Hosny A. E. Ibrahim1, Mohamed A. Hashem2*, Nagah E. Mohamed1, Aliaa A. Abd El-Rahman3
1Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; 2Clinical Pathology Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; 3Veterinary Directorate, Zagazig, Sharkia Province, Egypt.
Abstract | The present study was aimed to elucidate the anti-diabetic effects of Zingiber officinale (ginger) and Nigella sativa (black seed) ethanolic extract on streptozotocin (STZ) - nicotinamide (NA) induced diabetic rats and compared the extract efficacy with metformin, a known as antidiabetic drug. Fifty male albino rats of Wistar strain were randomly divided into five equal groups; the 1st group was kept as normal control, and 2nd to 5th groups were injected with STZ to induce diabetes. The 2nd group was left without treatment as positive diabetic control, while the 3rd to 5th groups were treated orally with metformin, ethanolic extract of ginger or N. sativa, respectively for 21 consecutive days. At the end of study, blood and pancreatic tissue sample were collected.The ethanol extract of either plant significantly improved the diabetic effects of STZ, specifically improved blood glucose level, serum fructosamine, and decreased serum insulin. Moreover, the changes in serum lipid profile, tissue antioxidants and various histopathological lesions in the pancreas caused by STZ- induced diabetes were significantly reduced. Plants extract improves serum biochemical and histopathological adverse effects occurred by diabetes as compared with the reference drug, metformin.
Keywords | Ginger, Nigella sativa, Diabetes, Lipid profile, Antioxidants.
Received | October 07, 2019; Accepted | February 17, 2020; Published | September 05, 2020
*Correspondence | Mohamed A. Hashem, Clinical Pathology Department, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Egypt; Email: mhashem.vet@gmail.com
Citation | Ibrahim HAE, Hashem MA, Mohamed NE, El-Rahman AAA (2020). Assessment of ameliorative effects of zingiber officinale and nigella sativa on streptozotocin-induced diabetic rats. Adv. Anim. Vet. Sci. 8(11): 1211-1219.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.11.1211.1219
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Hashem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Diabetes mellitus (DM) is a heterogeneous systemic metabolic disease. It is described as a hyperglycemia, glucosuria, with disturbances in carbohydrate, fat and protein metabolism caused by insulin secretion defects, insulin action, or both (Ali Sangi and Al Jalaud, 2019).
Recently, administration of nicotinamide (NA) in a suitable dose before STZ administration was used for experimental induction of type 2 diabetes in rats. Injection of NA leading to partial beta –cells protection from necrosis resulted from STZ injection (Lee et al., 2010). This model may give beneficial tool especially for new insulinotropic agents’ investigations (Madkor et al., 2011). The commonly anti-diabetic drug formerly used is metformin. It is used to treat T2DM (Alshathly, 2019).
On contrary, hundreds of conventional folk medicines have been used to treat diabetes with less side effects and tolerability. Thus, there is an increasing need to search for more natural antidiabetic agents from traditional medicine.
Zingiber officinale belongs to the family Zingiberaceae, has staring impending for treating a number of ailments including DM, cardiovascular, and digestive disorders, vomiting, and cancer (Mashhadi et al., 2013), because it contains more chemical compounds, such as (6)- gingerol, α-zingiberene, phenolic compounds, essential oils, and oleoresin resins (Kondeti Ramudu et al., 2011).
Nigella sativa of the family Ranunculaceae is infamous to have valuable effects in the handling of lots of diseases and this correlated to plentiful active components which have been isolated from seeds and its oil including dithymoquinone, thymoquinone, thymol, nigellimine-N-oxide, carvacrol, nigellicine, and flavonoids (Randhawa and Alghamdi, 2011; El Rabey et al., 2017).
Hence, the aim of the present study was to ascertain therapeutic potential of Zingiber officinale and Nigella sativa ethanolic extract as compared with metformin, a reference drug, in diabetic-associated disorders in an animal model.
MATERIALS AND METHODS
Chemicals and Reagents
Streptozotocin (STZ) and Nicotinamide (NA) have been obtained from the Sigma Chemicals (USA) and used for induction of the diabetes. All additional chemicals that were used in the study were of standard analytical grade. Metformin hydrochloride ((Glucophage®) hydrochloride, a product of Minapharma Co., Ltd., Egypt) was dissolved in saline or sodium chloride (0.9%).
Plants and Extract Preparation
Fresh ginger roots and N. sativa seeds were obtained from a local herbal market in Zagazig city, Egypt. The plants were identified in the Pharmacognosy Department, Faculty of Pharmacy, Zagazig University, Egypt. In an electrical grinder, the air-dried plants were finely powdered and stored at 5°C until further use. One kilogram of ginger or N. sativa powdered materials was mixed with 2 liters of ethyl alcohol 95% at room temperature and filtrated by Whatman filter paper (No .1). Finally, the filtrates were evaporated in a Soxhlet evaporator at 40°C for removal of alcohol. The yield residue (around 20-25 g) was held in glass containers which were firmly closed. It was kept at −20 °C and dissolved in corn oil before use.
Experimental Animals
Fifty healthy adult male Wistar rats, weighing 200–250g, were attained from Laboratory Animal Unit, Faculty of Veterinary Medicine, Zagazig University, Egypt. The rats were residing under perfect hygienic environment in the prepared metal cages, provided balanced feed with water ad-libitum and observed for 7 days before the experimental procedures began. All procedures of the current experiment were carried out in accordance with the Egyptian laws and university guidelines for the care of experimental animals and have been approved by the Committee of the Faculty of Veterinary Medicine, Zagazig University, Egypt.
Induction of Diabetes
For the induction of diabetes mellitus type 2, overnight fasted rats were intraperitoneally (i.p.) injected with nicotinamide (NA; 110 mg/kg b.wt., i.p.; dissolved in saline) to minimize the streptozotocin (STZ) induced pancreatic β-cell damage. Fifteen minutes later, STZ (55 mg/kg b.wt., i.p.) dissolved in 0.1 M citrate buffer (pH: 4.5) was injected for category II diabetes induction (Adam et al., 2017). Experimental animals were given glucose solution (5%, w/v (orally on the 1st day after STZ administration to starve off hypoglycemic shock. After two days from STZ injection, the fasting blood glucose level in blood samples obtained from the retro-orbital venous plexus was determined using a portable glucometer (Accu – sure, Roche Diagnostics, USA). Only rats with fasting blood glucose ≥ 250 mg/dl were considered diabetic and included in the experiment.
Experimental Design
The rats were haphazardly allocated into five equal groups.
Group 1: Normal rats received saline solution, used as a negative control.
Group 2: Diabetic rats were used as a positive control.
Group 3: Rats with diabetes were given metformin 100 mg per kg b.wt. (Choudhari et al., 2017).
Group 4: Rats with diabetes were received ginger (200mg/kg b.wt.) (Bhandari et al., 2005).
Group 5: Rats suffered from diabetes were supplemented N. sativa (300mg/kg b.wt.) (Asaduzzaman et al., 2015).
Metformin, ginger and N. sativa extracts were given once daily via oral gavage for 21days.Treatments were started on the day 3 after STZ injection, and were regarded day 1 for treatment and sustained for 21 days.
Sampling
In all experimental rats the fasting blood glucose was estimated initially in samples (one drop of blood ) obtained from the retro-orbital venous plexus on the 2nd and 4th day and once a week thereafter throughout the study period to decide the diabetic status. All rats were starved overnight for 12h at the end of study (21 days), and then sacrificed under light ether anesthesia for blood and tissue collection.
Blood was collected in non-heparinized tubes, allowed to clot at room temperature and then centrifuged for 10 minutes at 3000 rpm (centrifuge, model C60 Hospitex Diagnostic, Italy). The sera were separated and stored at −20°C for a week before the biochemical tests.
Tissue samples from each rat’s pancreas were quickly excised and trimmed from surrounding tissue then washed with sodium chloride 0.9 per cent and distilled water. They were then blotted over a piece of filter paper. Half grams of each tissue were infused with a 50 mM sodium phosphate buffer saline (100 mM Na2HPO4/NaH2PO4) (PH 7.4) containing 0.1 mM (EDTA) in an ice-containing medium to remove any clots and/ or red blood cells. Then tissues homogenates were prepared by homogenizing pancreatic tissue in 5–10 ml cold buffer per gram tissue and centrifuged for 30 min at 3000 rpm. The resulting supernatant was conveyed into Eppendorfs, and held at -80ºC in liquid nitrogen until it was used for antioxidant /oxidant assays. Other parts from pancreatic tissues were fixed in neutral formalin solution (10%) for histopathological examination.
Biochemical Analysis
The serum samples were assayed for the biochemical parameters including a diabetic markers (glucose, fructosamine and insulin), and lipid profile [cholesterol, triacylglycerides, and different lipoprotein fractions]. Antioxidant enzymes [superoxide dismutase (SOD) and catalase (CAT)], and oxidative stress biomarkers (e.g. MDA, malondialdehyde) were determined in pancreatic tissue. The assessments previously described have been carried out in the Central Laboratory (Faculty of Veterinary Medicine, Zagazig University, Egypt) using standard methods.
Histopathological Study
The formalin preserved pancreatic tissues were dehydrated, and embedded in paraffin. Paraffin blocks were sectioned at approximately 3-5 μm thickness and stained with hematoxylin and eosin (H&E) for examination of any histopathological changes.
Statistical Analysis
All data in this experimental study were subjected to one-way ANOVA, followed by Duncan’s Multiple Range post hoc test [Statistical Program of Social Sciences (SPSS) of version 17, Chicago, USA], with p < 0.05 being regarded as significant.
RESULTS
Effects of Stz- Induced Diabetes on Diabetic Markers
A blood glucose level at different periods among all experimental groups was shown in Table 1. Remarkable augmentation in blood glucose values (p < 0.001) was noted in STZ- induced diabetic rats (gp. 2) matching with normal control (gp.1). The ginger ethanolic extract-treated rats (gp. 4) illustrated a diminution in sugar concentrations matched with the second group. Moreover, in the metformin-treated rats (gp. 3), the blood glucose concentration decreased statistically (p < 0.01) after 21 days of the experiment, in comparison to STZ- induced diabetic group. Whereas supplementation of STZ- injected rats with N. sativa extract produced non significant decrease in glucose level if matched with gp. (2).
A significant rise and diminution in serum fructosamine and insulin levels respectively were noted in the animals of STZ- diabetic group when matched with the 1st group (Table 2). Rats of the 3rd – 5th groups that supplemented with metformin, ethanolic extracts of ginger or N. sativa displayed a significant decrease in serum fructosamine level at the end of the experiment matching with gp. (2). However, the level of serum insulin was statistically (p< 0.01) increased after 21 days in rats of groups (2-5).
Effects of Stz- Induced Diabetes on Lipid Profile
The results of lipid profile in all groups were presented in Table 3. Intraperitoneal injection of streptozotocin/ nicotinamide resulted in high concentrations of total cholesterol, triglycerides, LDL-C and a low HDL-C value in parallel to the normal rats (gp.1). Lipid profile were statistically declined on 21days post- supplementation of rats with metformin, ethanol extract of ginger or N. sativa (gps. 3-5). The results in metformin- treated group were better than others. However, serum HDL-C was augmented in all supplemented diabetic rats.
Effects of Stz- Induced Diabetes on Antioxidants and Oxidant Stress Markers
STZ- injected rats (group 2) showed significantly (p<0.001) lower CAT and SOD activities, and significantly (p <0.001) higher MDA levels in the pancreatic tissue than in the first group (Table 4). The aforementioned tissue antioxidants were significantly improved and restored towards normal after 21days post- supplementation with metformin, and ethanolic extract of ginger or N. sativa. However, tissue MDA was significantly diminished matching with that in untreated diabetic rats (gp. 2). Treatment results with metformin were better than those with plants extract.
Effects of Stz- Induced Diabetes on Histopathology of the Pancreas
Microscopical examination of pancreatic tissue of normal control rats (gp. 1) revealed that the islets of Langerhans as well as circumscribed masses surrounded by deeply stained pancreatic exocrine acini appeared normal (Fig.1 A, B). β- cells could be identified partially with their location and their smaller dark nuclei, relative to those of α-cells. The cell clusters were separated by thin-wall blood sinusoids. The pancreatic section from diabetic rats (gp. 2) revealed reduction in size due to hypocellularity, some of them suffered apoptosis or necrosis which was represented by more eosinophilic cytoplasmic or eosinophilic globules in the cytoplasm and shrinkage of the nuclei and other islets cells showed pyknotic or karyorrhectic nuclei (Fig.1 C, D, E). Intense interlobular fibrosis and edema admixed with inflammatory cells and fibroblasts were seen (Fig.1 F). Common pancreatic duct showed hyperplastic epithelium surrounded with chronic inflammatory reaction (mainly lymphocytes and plasma cells) rich in fibroblast (Fig.1G). Diabetic animals treated with metformin (gp. 3) revealed a decline in the number of cellular contents with a pyknotic and or necrotic nuclear changes. Some of the islets showed marked reduction in the cellular contents, especially β-cells.
Table 1: Effect of oral administration of metformin (100mg/kg b.wt.), ginger (200mg/kg b.wt.) or N. sativa (300mg/kg b.wt.) on blood glucose levels in STZ- induced diabetic rats. (Mean ± SE) (n=5).
|
Items |
Blood glucose level (mg/dl) |
||||
|
2nd day |
4th day |
1st week |
2nd weeks |
3rd weeks |
|
| Gp. 1 (Normal control) |
122.40 ± 9.07c |
117.40 ± 9.79c |
112.40 ± 9.45c |
104.60 ± 1.86b |
105.20 ± 2.96b |
| Gp.2 (Diabetic control, DC) |
451.00± 54.98a |
451.00± 14.65a |
264.40±28.38ab |
350.00± 35.59a |
232.40± 22.89a |
| Gp.3 (DC + metformin) |
389.20±16.67ab |
278.80± 20.30b |
196.60±12.49bc |
181.00± 19.44b |
99.00 ± 4.76b |
| Gp.4 (DC + ginger) |
344.40±18.25ab |
309.00± 11.98b |
131.00 ± 3.11c |
118.20 ± 8.73b |
105.20 ± 4.87b |
|
Gp.5 (DC + N. sativa) |
418.20± 26.06a |
383.40±25.37ab |
326.40± 29.45a |
309.00± 28.62a |
201.60± 14.91a |
Mean within the same column in each category carrying different superscripts are significant at p ≤0.05.
Table 2: Effect of oral administration of metformin (100mg/kg b.wt.), ethanol extract of ginger (200mg/kg b.wt.) or N. sativa (300mg/kg b.wt.) on serum fructosamine and insulin levels in STZ-induced diabetic rats. (Mean ± SE) (n=5).
|
Items
|
Fructosamine (µmol/l) |
Insulin (μU/ml) |
| Gp. 1 (Normal control) |
101.37 ± 1.82e |
47.61 ± 0.79a |
| Gp.2 (Diabetic control, DC) |
370.24 ± 1.44a |
5.63 ± 0.46e |
| Gp.3 (DC + metformin) |
198.69 ± 5.25d |
29.82 ± 2.35b |
| Gp.4 (DC + ginger) |
317.48 ±17.79b |
13.64 ± 0.72d |
| Gp.5 (DC + N. sativa) |
273.20 ± 6.39c |
21.65 ± 1.78c |
Mean within the same column in each category carrying different superscripts are significant at p ≤ 0.05.
Table 3: Effect of oral administration of metformin (100mg/kg b.wt.), ethanol extract of ginger (200mg/kg b.wt.) or N. sativa (300mg/kg b.wt.) on serum lipid profile levels in STZ-induced diabetic rats. (Mean ± SE) (n=5).
|
Items |
Lipid profile concentrations (mg/dl) |
||||
| Total cholesterol | Triglycerides |
HDL-C |
LDL-C |
VLDL-C |
|
| Gp. 1 (Normal control) |
53.69 ± 0.875e |
75.74 ± 1.288e |
38.75 ±0.502a |
11.75 ± 0.981d |
15.14 ±0.257d |
| Gp.2 (Diabetic control, DC) |
177.88 ± 3.754a |
208.44 ± 4.089a |
4.98 ± 0.247e |
216.65 ±4.554a |
41.68 ±0.818a |
| Gp.3 (DC + metformin) |
108.23 ± 2.682d |
120.73 ± 4.487d |
21.58 ±2.256b |
141.89 ±1.274c |
25.94 ±0.898c |
| Gp.4 (DC + ginger) |
153.25 ± 2.156b |
182.03 ± 2.223b |
11.68 ±0.656d |
175.97 ±2.674b |
34.40 ±0.445b |
|
Gp.5 (DC + N. sativa) |
133.99 ± 2.597c |
156.53 ± 3.347c |
16.59 ±0.940c |
141.89 ±1.278c |
31.30 ± 0.669b |
Mean within the same column in each category carrying different superscripts are significant at p ≤ 0.05.
HDL-C, high density lipoprotein- cholesterol; LDL-C, low density lipoprotein- cholesterol; VDL-C, very density lipoprotein- cholesterol
Table 4: Effect of oral administration of metformin (100mg/kg b.wt.), ethanol extract of ginger (200mg/kg b.wt.) or N. sativa (300mg/kg b.wt.) on pancreatic CAT, SOD and MDA tissue levels in STZ-induced diabetic rats. (Mean ± SE) (n=5).
|
Items
|
Antioxidants / Oxidative Status |
||
|
CAT (Umol/mg protein) |
SOD (U/mg protein) |
MDA (nmol/mg protein) |
|
| Gp. 1 (Normal control) |
0.49± 0.023a |
36.04±0.317a |
84.80 ± 1.41d |
| Gp.2 (Diabetic control, DC) |
0.09 ± 0.010e |
2.02 ± 0.295e |
193.80 ± 1.39a |
| Gp.3 (DC + metformin) |
0.32 ± 0.012b |
19.44 ± 2.16b |
132.20 ± 1.98c |
| Gp.4 (DC + ginger) |
0.16± 0.008d |
7.55 ± 0.531d |
171.20 ± 2.07b |
|
Gp.5 (DC + N. sativa) |
0.25±0 .009c |
12.77±0.914c |
171.20 ± 2.08b |
Mean within the same column in each category carrying different superscripts are significant at p ≤ 0.05.
CAT, catalse; SOD, superoxide dismutase, MDA, malondialdehyd
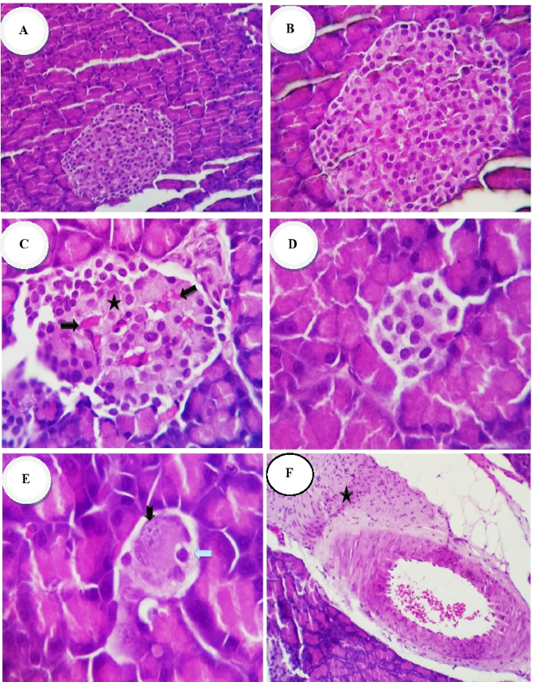
Figure 1A-F: Photomicrographs of the rat’s pancreases of Islet of Langerhans (H and E stain): Group 1 (normal control) showed: (A, B) normal size of islet of Langerhans and Cellularity x200 and x400 respectively. Group 2 (diabetic control) showed: (C) destruction or necrosis of islets (star), congestion of capillaries (thick arrow) with hypocellularity; (D, E) reduction in size, necrosis apoptosis, pyknosis and karyorrhectic of islet cells nuclei (arrows), x400; (F) intense interlobular fibrosis and edema admixed with inflammatory cells and proliferated fibroblasts (star), x200
cells. Other islets were rudimentary with a few cellular contents (Fig.1 H). Mild vascular dilatation, ductal dilation and infiltration of inflammatory cells were noted. Pancreatic sections from diabetic rats treated with ginger extract (gp. 4) revealed normal islets with average number and size of cellular contents besides mildly congested capillaries. In other cases some islets were moderately decreased in size and cellular contents (Fig.1 I). Mildly dilated pancreatic ducts with retention of the secretory contents could be also observed (Fig.1 J). Pancreatic sections from N. sativa – treated rats (gp. 5) revealed pyknotic changes (early necrotic changes) in islets cells and or vacuolation of the cytoplasm (Fig.1 K). Other cases showed completely normal numbers and morphology of islet cells with a mild interstitial dilatation of the blood vessels, and mild perivascular fibrosis (Fig.1 L).
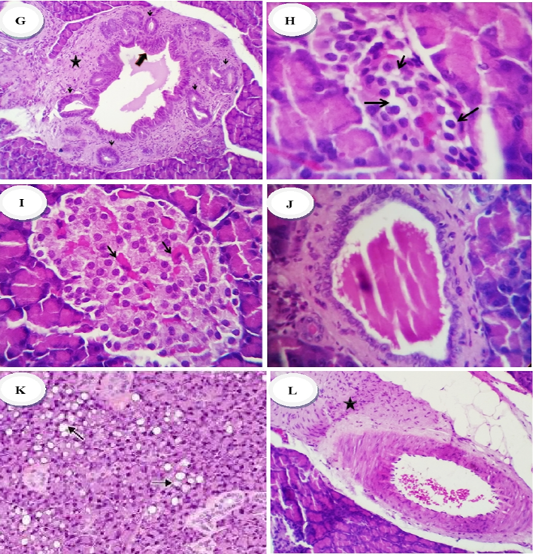
Figure 1G-L: Group 2 (diabetic control) showed hyperplastic epithelium (thick arrow) of commo duct surrounded with chronic inflammatory reaction (mainly lymphocytes and plasma cells) (star) rich in fibroblast (thin arrow), x400 (G) Group 3 (diabetic rats treated with metformin) revealed: (H) reduction in the number of cellular contents especially β-cells with a pyknotic an/or necrotic nuclear changes, x200, Group 4 (diabetic rats treated with ethanolic extract of ginger) showed: (I) normal islets with average number and size of cellular contents, besides mildlu congested capillaries (arrows), (J) mildly dilated pancreatic ducts with retention of the secretory contents, x400. Group 5 (diabetic rats treated with ethanolic extract of N.sativa) showed: (K) islets with pyknotic changes (early necrotic changes), x400 and or vacuolation of the cytoplasm (arrows), (L) perivascular fibrosis (star), x200
DISCUSSION
DM is a chronic condition, marked by a rise in glucose in blood and urine (Karim et al., 2020). In this study, type 2 diabetes was induced by intraperitoneal injections of STZ and NA. It was previously reported that STZ-NA had a diabetogenic effect of (Lee et al., 2010). STZ is one of the most collectively used substances to induce diabetes in rats. This toxin induces death of pancreatic cells by alkylation with DNA, leading to reduced DNA synthesis and insulin release (Seedevi et al., 2020). Moreover, STZ enters the pancreatic β-cells through glucose protein-2 transporter and interferes with the equilibrium between oxidant and antioxidant systems, destroying the insulin-producing islet β-cells and allowing diabetes to progress.
Administration of STZ in the present study is able to raise blood glucose levels which may be due to the destruction of pancreatic islets with death of β-cells. The present hyperglycemia is confirmed by statistically increases in serum fructosamine and decreases of insulin levels, and histopathological alterations of pancreatic islets. A different literature confirmed that STZ induced damage to islet β-cells, which in effect increased and declined sugar and insulin values, respectively (Al-Attar and Alsalmi, 2019; El-Baz et al., 2020). Diabetic groups given metformin, ethanolic extracts of ginger or N. sativa exhibited a statistical diminution in glucose & fructosamine values, in addition to a rise in level of insulin when matched with gp. 2. However, blood glucose didn’t decrease to base line level in rat’s supplemented N. sativa (gp.5). Results were more prominent with metformin administration than that of plants. Therefore, this suggests that metformin and ginger produced better glycemic control. This observation was supported by a previous study in rats (Tzeng et al., 2013). Metformin has been regarded as sensitizer to insulin (Wulffelé et al., 2004), drop off blood glucose through inhibition of gluconeogenesis and increases consumption of glucose in liver, intestine, and muscle (Birgani et al., 2018) and displays efficiency in reducing diabetes type 2 in mice (Lee et al., 2010). Ginger’s hypoglycemic action may be attributable to actions involving serotonin receptors, an increase in pancreatic insulin secretion from β-cells or release of bound insulin (Al-Amin et al., 2006), promotion of glucose clearances in insulin-responsive peripheral tissues and augmented insulin release (Li et al., 2012) or inactivation of free radicals (Bhandari et al., 2005). However, other study showed that oral treatment with N. sativa ethanolic extract produced a significant decline in the elevated sugar concentration; with an enhanced the antioxidant status of STZ- induced diabetes in rats (Kaleem et al., 2006). The anti-diabetic effect of N. sativa possibly clarified by insulin-like stimulation of glucose uptake by adipose tissue, and muscle (Benhaddou-Andaloussi et al., 2010), hindrance of intestinal sugar assimilation (Meddah et al., 2009) or could be due to amelioration of β-cell ultrastructure, thus leading to increased insulin levels (Abdelmeguid et al., 2010; Asaduzzaman et al., 2015).
Lipids play a key function in DM pathogenesis. An abnormality in the lipid profile contributes to secondary complications of diabetes (Karim et al., 2020). In the existing study, the lipid profile exhibited an augmentation in serum levels of cholesterol, triglycerides, lipoproteins of low and very low density and decreased HDL-C in rats of gp. (2). The hyperlipidemia induced by STZ injection was attributed to excess mobilization of fat from the adipose tissue owing to the underutilization of glucose (Choudhari et al., 2017). The observed findings are consistent with other published reports (Rajasekaran et al., 2006; Adam et al., 2017). Unlike normal rats, non- significant differences in the serum lipid profile were observed in rats injected with STZ (40 mg/kg b.wt)- induced diabetes (Balamash et al., 2018). The dissimilarity may be recognized to different dose or method of diabetes induction.
Metformin or ethanol extracts administration had favorably modified serum lipogram results in STZ- diabetic rats due to their ameliorative effects on the abovementioned findings induced by streptozotocin/nicotinamide. The results may be owing to diminutive cholesterol biosynthetic enzymes activity or the insignificant level of lipolysis under the control of insulin (Seedevi et al., 2020). The effect was better in lipid profile with metformin administration. But the effect of ethanolic extract of N. sativa was better than that of ginger. Previous studies confirm the favorable mechanism of metformin on lipid profile by either improved insulin secretion or insulin sensitivity (Al-neaimy, 2011; Garimella et al., 2016; Wen et al., 2019). Ginger’s hypo-lipidemic action was due to a drop off in biosynthesis of cholesterol and in superior hepatic uptake of circulating LDL- C, or amplified activity of LDL-c receptor resulting in higher LDL-c removal and lower cholesterol level (Li et al., 2012). A variety of inquiries stated that Z. officinale ethanol extract alleviates plasma lipids in cholesterol- fed hyperlipidemic rabbits (Verma et al., 2004) and in STZ-injected rats (Nammi et al., 2009; Kondeti Ramudu et al., 2011; Jiyil et al., 2019). Administration of N. sativa seeds extract improved considerably serum lipids which were however not completely normal. The hypo-lipidemic effect of N. sativa may be endorsed to the synergistic effect of its various constituents, soluble fiber, sterols, flavenoids and high content of polyunsaturated fatty acids (El-Bahr et al., 2014), and coincide with aforesaid studies (Kocyigit et al., 2009; Asaduzzaman et al., 2015).
Oxidative stress plays a role in diabetes development, while antioxidants help diabetes alleviation. The oxidative status marker (MDA, a measure of lipid peroxidation) was significantly elevated in this study, with a significant diminution in activities of CAT and SOD enzymes in pancreas of diabetic rats. Hyperglycemia in diabetes results in free radicals generation; this weakens the body’s defense mechanisms, thus leading to the disruption of cellular functions, oxidative membranes damage and lipid peroxidation augmentation. The present findings and comments are in parallel with earlier observations (Rondi et al., 2014; Adam et al., 2017; Althnaian et al., 2019).
Supplementation of ginger and N. sativa ethanolic extracts or metformin to rats with diabetes dramatically enhanced the levels of antioxidant markers as they significantly reduced the diabetes-induced increases in tissue MDA and significantly increased tissue CAT and SOD. The aforesaid results signifying the antioxidant characters of ethanol extract in diabetes-mediated complications. The effect of ginger administration was depended upon its capability to restrain lipid peroxidation and hence protect β- cells from diabetic free radicals damaging effect (Mashhadi et al., 2013). The phenolics and flavonoids contents of N. sativa extract may possibly be an explanation for their antioxidant activity which have a scavenging achieve on the free radicals (Kanter et al., 2004; Althnaian et al., 2019). The observed findings are consistent with other published reports (Kaleem et al., 2006; Abdelmeguid et al. 2010; El Rabey et al., 2017). N. sativa administration exerts a useful effect in diabetes by lessening pancreatic morphological changes and conservation β-cell integrity thus defending beta cells against oxidant burden (Kanter et al., 2009; Asaduzzaman et al., 2015).
The obtained findings were supported by pathological alterations in pancreatic sections from diabetic control rats (gp. 2) which showed a reduction in size of the endocrine islets of Langerhans with β-cells, degenerative and necrotic changes, and hyperplastic epithelium surrounded with chronic inflammatory reaction in the common ducts. Other islets cells showed pyknotic or karyorrhectic nuclei, with intense interlobular fibrosis and edema admixed with inflammatory cells. The histopathological changes observed in the diabetic group were like those reported previously (Nurdiana et al., 2017; El-Baz et al., 2020). The pancreatic histopathology observed in diabetic rats was ameliorated by treatment with metformin, ethanolic extracts of ginger and N. sativa. The majority of the cells in pancreas tissues restored the normal conditions. This observation has already been reported by several investigators (Balamash et al., 2018; Wen et al., 2019).
CONCLUSION
The present study corroborated the beneficial effects of ginger or N. sativa in attenuating the alterations in serum biochemical parameters and histopathology cellular damage in the pancreas due to exposure to STZ, as compared with the reference drug, metformin.
ACKNOWLEDGMENTS
Grateful thanks are given to Dr. Elsayed Rashad El-Attar, Professor of Pathology, Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, for his effort in the pathological examination.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Hosny A. E. Ibrahim and Nagah E. Mohamed conceived and designed research. Aliaa A. Abd El-Rahman carried out all experiments and performed data analysis. Mohamed A. Hashem and Aliaa A. Abd El-Rahman drafted the manuscript, made critical contribution to the discussion and revised the manuscript. All authors read and approved the final manuscript.
REFERENCES






