Advances in Animal and Veterinary Sciences
Research Article
Evaluation Of Biomarkers Distress In Acrylamide-Induced Hepatic And Nephrotoxicity Of Albino Wistar Rat
Sreenivasulu Dasari1*, Muni Swamy Ganjayi1, Sailaja Gonuguntla1, Srinivasa Rao Kothapalli2, Prabhakar Yellanur Konda3, Sreenivasulu Basha4, Peera Kutagolla5, Balaji Meriga1
1Department of Biochemistry, Sri Venkateswara University, Tirupati-517 502, A. P., India; 2Department of Veterinary Parasitology, College of Veterinary Science, Sri Venkateswara Veterinary University, Tirupati-517 502, A. P., India; 3Biochemistry Division, National Institute of Nutrition, Hyderabad, India; 4Department of Microbiology, Sri Venkateswara University, Tirupati-517 502, A. P., India; 5Department of Zoology, DBT BIF Center, Sri Venkateswara University, Tirupati-517 502, A. P., India.
Abstract | Disturbance of the biomarkers in hepato and nephrotoxicity that induced by acrylamide (ACR) were studied in rat. ACR (50 mg) was administered through drinking water (300 ml) on alternative days up to 27 days, and liver and kidney tissues were collected on 27th day post ingestion for experimentation. The results revealed that ACR causes significant increase in non-enzyme markers such as lipid peroxidation (p˂0.05) and nitric oxide (NO) (p˂0.05), but decrease in glutathione (GSH) (p˂0.05), and similarly enzymemarkers such as glutathione peroxidase (GPx) and glutathione S-transferase (GST) activities were significantly decreased (p˂0.05) at 27th day post ingestion. The histology study revealed that ACR induced degenerative changes in the liver and kidney such as enlarged sinusoid, fragmentation of hepatocyte nuclei, inflammation of Kupffer cells and spreading hepatic contents towards central vein, and tubular thyroidisation, inflammation in distal tubules, distorted glomerulus and proximal tubules fragmentation, respectively. In conclusion, ACR that shows significant effect on liver and kidney biomarkers and leads to liver and kidney tissue degeneration wistar rats.
Keywords | Acrylamide, Chemo-stress markers, Liver and kidney degeneration
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 06, 2018; Accepted | August 31, 2018; Published | September 21, 2018
*Correspondence | Sreenivasulu Dasari, Department of Biochemistry, Sri Venkateswara University, Tirupati-517 502, A. P., India; Email: dasarisreenivaasulu@gmail.com
Citation | Dasari S, Ganjayi MS, Gonuguntla S, Kothapalli SR, Konda PY, Basha S, Kutagolla P, Meriga B (2018). Evaluation of biomarkers distress in acrylamide-induced hepatic and nephrotoxicity of albino wistar rat. Adv. Anim. Vet. Sci. 6(10): 427-435.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.10.427.435
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Dasari et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Acrylamide (ACR) become a major public health concerns when it has detected in the daily consuming food products like fried bread, potato chips and all carbohydrate-rich foods those cooked at high temperature (i.e. ˂ 200оC) (Guyton and John, 2006; Kaneko et al., 2008), chips/French fries, crisps and bread, biscuits, crackers and breakfast cereals (Tareke et al., 2002), using in several fields including laboratories and it absorbed while occupational exposure (Meng et al., 2001; Boettcher et al., 2005), it causes to generation of free radicals and hydro-peroxides which leads to lipid peroxidation (Prasad, 2012). The free radicals are the main reason for pathogenesis of many diseases like diabetes, diseases of cardiovascular system, organs degeneration, neoplasm formation (Rahman et al., 2012; Gren, 2013). Because the continuous invading of the toxic chemicals like AC made tissue injury (Dasari et al., 2017a).
The lipid peroxidation has associated with the free radicals which promote cellular damage. Serum malondialdehyde (MDA) is a final product of peroxidation of polyunsaturated fatty and that used as marker of oxidative stress (Irmak et al., 2003). The thiobarbituric acid reactive substances (TBARS) were systematically increased in rat that ingested by ACR through oral (Yousef and El-Demerdash, 2006). Nitric oxide is a chemical mediator that is an integral part in the maintenance of physiological homeostasis due to its regulatory and protective functions. The NO is produced by numerous cells that include the immune system, which show the systemic action on different organs, tissues and tumor cells (Moncada ad Higgs, 1995; Singh and Gupta, 2011).
Glutathione (GSH) is a tri-peptide (γ-Gln-Cys-Gly) that serves as a major antioxidant, but ACR causethe depletion of GSH hence redox imbalance occurs. Generally, consumption of the GSH occurs at high level with the reactions of hydrogen peroxide (Zhu et al., 2008; Kopanska et al., 2015) and conjugation with ACR and glycidamide (ACR metabolite) that reaction catalysed by glutathione S-transferase (GST) (Paulsson et al., 2005). The reduced glutathione play key role in neutralization of free radicals by donating their electrons (Lobo et al., 2010). GSH is abundant in cells of all organs and play major role in protection of the cell from oxidative stress (Gawryluk et al., 2011), it is recycled from oxidized state (GSSG) to reduced state (GSH) (antioxidant form) by Glutathione reductase (GR) and it is up-regulated in oxidative stress (Gawryluk et al., 2011; Schuliga et al., 2002).
Five selenium-dependent Glutathione peroxidase (GPx) isoforms are abundant mammalians, but non-selenium GPx contains selenocysteine (Brigelius-flohe and Maiorino, 2013). GPx present in both cytosol and mitochondria and isoenzymes of GPxfamily catalyze the reduction of H2O2 and lipid peroxides by using GSH (Lin and Beal, 2006; Gandhi and Abramov, 2012; Dasuri et al., 2013). Glutathione S-transferases (GSTs)[EC 2.5.1.18] are widely distributed in both prokaryotes and eukaryotes and they belongs to phase II detoxification enzymes in eukaryotes (Hayes et al., 2005), they protect cellular macromolecules from reactive oxygen species (ROS), environmental carcinogens and chemotherapeutic drugs (Strange et al., 2000), they catalyse the nucleophilic addition of GSH to electrophilic center of numerous toxic chemical agents (xenobiotics) and carcinogenic metabolites which generated by phase I enzymes (Senhaji et al., 2015; Nebert and Vasiliou, 2004; Hayes and Strange, 2000). GST exhibit the multiple biological functions like xenobiotic detoxification, removal of oxidative stress products, transport of protein, modulation of cell proliferation and induction of the apoptosis signalling pathway (Laborde, 2010). Detoxification by GST is beneficial to the cells because it regenerates stress free environment in the cell (Dasari et al., 2018a).
The cytotoxicity occurs because of the damaged mitochondria in the tubules, hence the tubular transport system is disturbed and the increased oxidative stress by free radical generation (Markowitz and Perazella, 2005), and excretion of the toxic chemicals and drugs does not take place smoothly in nephrotoxicity (Finn and Porter, 2003). The decrease of total protein is an index of serious damage of cellular function in chronic liver diseases as well as severe alterations of the liver tissue (El-Bohi et al., 2011; Yousef and El-Demerdash, 2006). The present study was aimed to investigate the liver and kidney biomarkers and their tissue degenerative changes in ACR ingested wistar rats.
MATERIALS AND METHODS
Chemicals
Acrylamide (ACR) (98% purity), thiobarbituric acid (TBA), glutathione (GSH), 5, 5’-dithiobis (2-nitrobenzoic acid) (DNTB), n-butanol, pyridine and nicotinamide adenine dinucleotide phosphate (reduced) tetrasodium salt (NADPH) were purchased from HiMedia laboratories Pvt. Ltd. Mumbai, India. Griess reagent was obtained from SRL, Mubai, India. All other chemicals (analytical grade) were purchased from standard chemical company (India).
Maintenance of Rats
Twelve male Wistar rats weighing about 200 grams were selected for experimentation and they were allowed to acclimatization for about one week. Throughout the experimental period they were housed plastic cages under standard laboratory conditions that includes 12:12 light/dark photoperiod at 23 ± 2 ºC. Experimental rats were fed with ad libitum and tap water throughout the study.
Acrylamide (ACR) Administration
Selected rats were divided as control and treated groups and each group have six animals (n=6). ACR has administered in drinking water i.e. 50 mg ACR in 300 ml waterfor 24 hours and only 300 ml of water has given in the following 24 hours. In that way as showed in Figure 1, ACR has administered in alternative days up to 27 days (14 doses). Each animal has drunk 30-40 ml that means 5-6.65 mg per day. Only water (vehicle) has given to control group.
Determination of Protein
Protein content has measured by the method of Lowry et al. (1951) in both control and ACR treated liver and kidney samples.
Thiobarbituric Acid (TBA) Test for Lipid Peroxidation
According to Ohkawa et al (1979) that lipid peroxidation levels were estimated in ACR administered rat liver and kidney. Briefly, 10% liver and kidney tissue homogenate was prepared with 1.15% KCl and 0.2 ml 8.1% SDS and 0.1 ml 0.8% TBA were added to 0.1 ml homogenate. Made up them to 4 ml with distilled water and those tubes were incubated for 1 hour at 95оC and allowed them to cool. Distilled water 1 ml and n-butanol-pyridine mixture (15:1 v/v) 5 ml were added to cooled tubes and those contents were mixed thoroughly. Those tubes were centrifuged at 4000 rpm for 10 minutes at room temperature and finally organic layer was measured by spectrophotometer at 532 nm and the results were expressed as nmol MDA/h/g tissue. The TBA activity is depending on the lipid content in the sample.
Estimation of Nitric Oxide (NO)
Usually, in the aqueous solution that nitric oxide (NO) is rapidly degraded as nitrate and nitrite. The total nitrite was estimated by spectrophotometer at 540 nm by using Griess reagent according to Jablonska et al. (2007) in ACR administered rat liver and kidney. In the presence of cadmium the nitrate in the sample was reduced to nitrite and finally converted as nitric acid that has given color reaction with Griess reagent; NO products were expressed in μ moles.
Determination ofGSH
According to Kurtel et al. (1992) the GSH levels were determined in ACR administered rat liver and kidney. In this method, 0.5 ml of brain homogenate added to 1 ml of 100 mM Tris HCl (pH 8.2) buffer that containing 1% SDS and 2 mM EDTA and then this mixture was incubated at 25оC for 15 minutes. It was centrifuged to remove precipitate and 2.5 ml of 0.3 mM5, 5’-dithiobis (2-nitrobenzoic acid) (DNTB) was added and the reaction mixture was incubated at 37оC for 15 minutes. Finally, the absorbance was measured at 412 nm by using spectrophotometer. GSH levels were calculated with 13,000 M-1cm-1 as molar extinction coefficient and the results were expressed as μmol/g tissue.
GPx Activity Assay
According to Wendel (1981), glutathione peroxidase (GPx) activity assay was done in ACR administered rat liver and kidney. Assay buffer: 0.25 mM phosphate buffer (pH 7.0) containing 2.5 mM EDTA and 2.5 mM sodium azide (NaN3). The reaction mixture contains, assay buffer 1.8 ml, 100 μl GR, 100 μl GSH, 100 μl NADPH and 250 μg of enzyme source (20% homogenate of liver and kidney). The reaction was initiated by the addition of 100 μl CHP/H2O2and linear decrease in NADPH absorption at 340 nm was measured for 3 minutes; a blank was maintained without enzyme source. GPx activity was expressed in terms of nano moles of NADPH is oxidised/min/mg protein at room temperature and GPx activity was calculated using molar extinction coefficient i.e. 6.32×103 cm-1.
GST Activity Assay
Rat liver and kidney sample GST activity assay was done by the method of Habig et al. (1974). The reaction mixture consists of 1 ml of 0.3 M phosphate buffer (pH 6.5), 30 mM CDNB 100 µl, 30 mM GSH 100 µl and enzyme source 100 µl (20% homogenate of liver and kidney). This reaction mixture was made up to 3 ml with distilled water. An increase in absorbance was measured at 340 nm by using spectrophotometer. One unit of GST activity was defined either as formation one µ mole of 2,4 dinitrophenol-glutathione conjugate per minute or one µ mole of substrate consumed per minute and GST activity was calculated by using molar extinction coefficient i.e. 9.6 ×103 M-1cm-1.
Histopathology
According to Humason (1972) in both rat liver and kidney that administered with ACR, histological examinations were conducted. Briefly, collected brain tissues from both control and experimental rats were washed with physiological saline (0.9% NaCl) to remove blood and fat debris adheres to the brain. After fixation in 10% of formalin the tissues were allowed to process. In first step, the tissues were washed under running tap water to remove the fixative. In second step, the tissues were allowed for dehydration by a graded series of alcohol and the tissues were allowed to clear by using methyl benzoate and subjected to embed in paraffin wax. In third step, the tissue were subjected to cutting with 6 µ thickness and such sections allowed for staining with haematoxylin and eosin (H & E). In fourth step, the sections were mounted with Canada balsam and observed under light microscope.
Statistical Analysis
All the data related to this study and their results were calculated from three experiments and presented as the mean ± standard deviation (SD). Student t-test was performed to identify the acrylamide treated liver and kidney samples differed from the mean for the respective vehicle controls. That the differences between the experimental groups at the level of P <0.05 were considered as significant.
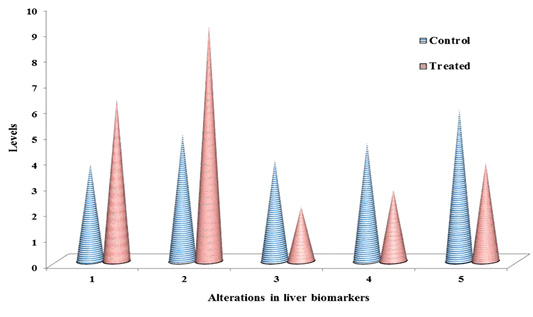
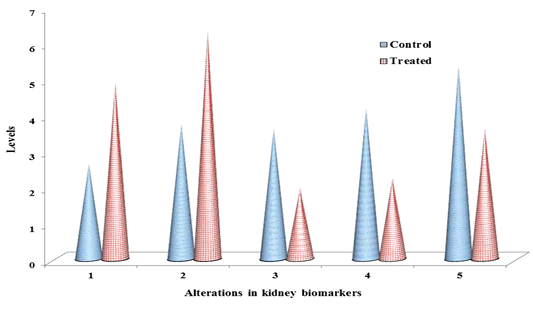
Table 1: ACR induced chemo-stress biomarkers modulation in liver & kidney.
| S.No | Biomarker | Liver control | Liver treated | Kidney control | Kidney treated |
| 1 | LPO | 3.81 ± 0. 57 |
6.35a ± 0.34 |
2.63 ± 0.23 |
4.91a ± 0.51 |
| 2 | NO | 4.97 ± 0.67 |
9.21a ± 0.21 |
3.76 ± 0.45 |
6.34 a ± 0.32 |
| 3 | GSH | 3.95 ± 0.45 |
2.13a ± 0.11 |
3.62 ± 0.32 |
1.97a ± 0.47 |
| 4 | GPx | 4.62 ± 0.51 |
2.79a ± 0.49 |
4.19 ± 0.18 |
2.25a ± 0.23 |
| 5 | GST | 5.93 ± 0.53 |
3.85a ± 0.53 |
5.37 ± 0.26 |
3.63a ± 0.42 |
Documented values represented in table 1, are average of three separate experiments of three samples. Mean ± standard deviation (SD). Student test (a = p<0.05, regarded as significance). ACR was administered by nine alternative days in drinking water (150 mg ACR/300ml). LPO: Lipid peroxidation, NO: Nitric oxide, GSH: Glutathione, GPx: Glutathione peroxidase, GST: Glutathione S-transferase. LPO: nmol MDA/h/g tissue, NO products: μ moles, GSH: μmol/g tissue, GPx: nM of NADPH oxidized/min/mg protein, GST: μM of GSH conjugate formed/min/mg protein.
Results
Chemo-stress Markers
The non-enzymatic biomarkers like lipid peroxidation (LPO), NO in terms of total nitrite and glutathione (GSH) levels as shown in Table 1 & Figure 2 & 3, and the enzymatic biomarkers like glutathione peroxidase (GPx) and glutathione S-tranferase (GST) levels as shown in Table 1 & Figure 2 & 3, were observed in ACR administered rat liver and kidney as follows:
LPO levels were significantly increased (p˂0.05) to 6.35 fold and 4.91 fold, inboth liver and kidney respectively than control i.e. 3.18 fold and 2.63 fold respectively, and NO levels in terms of total nitrite were significantly increased (p˂0.05) to 9.21 fold and 6.34, in both liver and kidney respectively than control i.e. 4.97 fold and 3.76 fold respectively, at 27th day post ingestion. But GSH levels were significantly decreased (p˂0.05) to 2.13 fold and 1.97 fold both in liver and kidney respectively than control i.e. 3.95 fold and 3.62 fold respectively, at 27th post ingestion.
With the substrate H2O2, GPx activity was significantly decreased (p˂0.05) to 2.79 fold and 2.25 fold both in liver and kidney respectively than control i.e. 4.62 fold and 4.19 fold respectively, and with the substrate CDNB the GST activity was significantly decreased (p˂0.05) to 3.85 fold and 3.63 fold both in liver and kidney respectively than control i.e. 5.93 fold and 5.37 fold respectively, at 27th day post ingestion.
Histopathological Studies
We observed the control liverand kidney sections with normal hepatocyte nuclei, kupffer cells, sinusoids and central vein, and proximal tubules, distal tubules, bowman’s space and glomerulus, respectively as shown in Figure 4 (A) and 5 (A). We observed the ACR induced histological changes in both liver kidney sections with enlarged sinusoid, fragmentation of hepatocyte nuclei, inflammation of Kupffer cells and spreading hepatic contents towards central vein, and tubular thyroidisation, inflammation in distal tubules, distorted glomerulus and proximal tubules fragmentation, respectively as shown in Figure 4 (B) and 5 (B) at 27th day post ingestion.
DISCUSSION
Both the liver and kidney are the vital organs, and they detoxify xenobiotic substances, but kidney only eliminates the detoxified xenobiotic products those soluble in water. Liver has several functions including the metabolism of drug and xenobiotic (Maton et al., 1997) but kidney that performs several functions including excretion of toxic metabolites and drugs (Ferguson et al., 2008). ACR that induce oxidative stress through the production of reactive oxygen species (ROS) cause suppression of cellular defence system hence the cells are easily susceptible to oxidative attack through altering the membrane integrity and fatty acid composition (Lee and Jacobs, 2005), mitochondria are the main target for the ACR (Zhao et al., 2015). During the production of cellular energy by aerobic metabolism simultaneously produce excessive toxic oxygen intermediates in terms of reactive oxygen species (ROS) which threat to cellular homeostasis (Cadenas and Davies, 2000), ROS are the main reason for the activation or deactivation of cellular signalling pathways while stress condition (Schieber and Chandel, 2014). The anti ROS cellular defence that mediated by enzymatic and non-enzymatic system is necessary to maintain normal cellular function (Valko et al., 2007), which necessary for the survival of organisms in stressful conditions (Vasseur and Leguille, 2004).
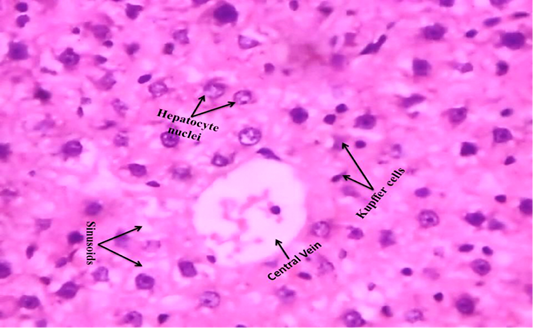
Figure 4A: Control rat liver shows that normal hepatocyte nuclei, kupffer cells, sinusoids and central vein (H & E stain) (40X)
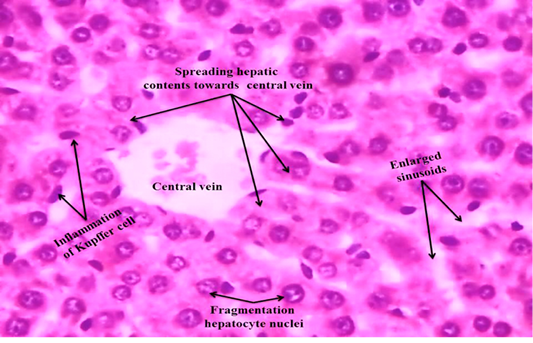
Figure 4B: ACR administered rat liver section shows that enlarged sinusoid, fragmentation of hepatocyte nuclei, inflammation of Kupffer cells and spreading hepatic contents towards central vein (H & E stain) (40X).
In the present study, as shown in Figure 1, ACR was administered in alternative days in drinking water (50 mg/300ml) to rat and evaluate the modulation of the non-enzymatic and enzymatic chemo-stress markers in liver and kidneyas shown in Table 1 and Figure 2 & 3 and also observed the tissue derangements as shown in Figure 4 (B) and 5 (B). ACR can enter in to the body through cigarette smoke, food, occupation, breast milk,water etc., hence distribute the entire the body. The oxidative stress is a process for excessive production of reactive oxygen species (ROS)/reactive nitrogen species (RNS) and depletion of antioxidant system (Zhu et al., 2008), imbalance between ROS/RNS and antioxidant system leads to apoptosis (Halliwell, 2006; Maiese et al., 2010).
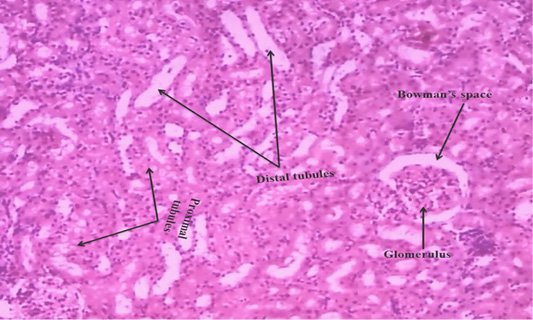
Figure 5A: Control rat kidney section shows that normal proximal tubules, distal tubules, bowman’s space and glomerulus (H & E stain) (40X).
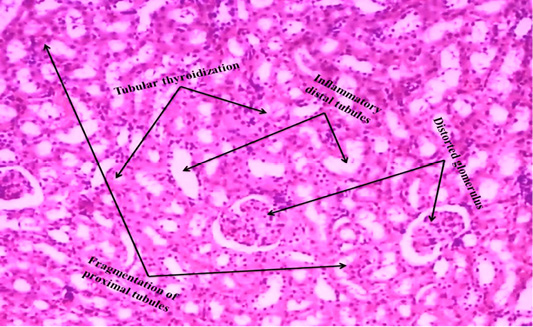
Figure 5B: ACR administered rat kidney section shows that tubular thyroidisation, inflammation in distal tubules, distorted glomerulus and proximal tubules fragmentation (H & E stain) (40X).
The levels of lipid peroxidation products were extensively measured in biological fluids and tissues of humans (Dotan et al., 2004). Lipid peroxidation cause changes the permeability and fluidity of the membrane lipid bilayer that can greatly alter the cell integrity (Dix and Aikens, 1993). Gutteridge (1995) reported the thiobarbituric acid reactive substances (TBARS), ethane and pentane, lipid hydroperoxides and malondialdehyde (MDA) were used as marker for the in vivo lipid peroxidation. In the present study, as shown in Table 1 and Figure 2 & 3, in ACR administered rat liver and kidney that LPO levels were significantly increased (p˂0.05) to 6.35 fold and 4.91 fold respectively than control i.e. 3.18 fold and 2.63 fold respectively, at 27th day post ingestion. In this study, we measured TBARS as marker to assess lipid peroxidation as stated by Gutteridge (1995).
Endothelial cells, platelets, neutrophils and neurons are release NO in response to homeostatic and pathologic stimuli (Ferrario et al., 1994). The NO is produced from inducible nitric oxide synthase (iNOS) that is apparently very important in host defence and chronic inflammatory response (Nussler and Billiar, 1993). In the present study, as shown in Table 1 and Figure 2 & 3, in liver and kidney the NO level in terms of total nitrite has significantly increased (p˂0.05) to 9.21 fold and 6.34 respectively than control i.e. 4.97 fold and 3.76 fold respectively, at 27th day post ingestion. The administration of high dose ACR (50 mg/kg bwt) has enhanced the NO production in terms of total nitrite (NO2–) in rat liver (Otzuran-Ozer et al., 2014)and we observed that similar result in ACR administered rat liver and kidney.
GSH is abundant in cells of all organs and involved in antioxidant reactions in two ways, non-enzymatically can it react with ROS like O2- and .OH- to be remove (Gandhi and Abramov, 2012). In this study, in both liver and kidney as shown in Table 1 and Figure 2 & 3, GSH levels were significantly decreased (p˂0.05) to 2.13 fold and 1.97 fold respectively than control i.e. 3.95 fold and 3.62 fold respectively, at 27th day post ingestion. In ACR administered rat the lipid peroxidation levels are increased and GSH levels are decreased (Mehhri et al., 2015). Both the TBARS levels and GST activity levels were increased in plasma, testes, kidney and brain,but GSH levels were decreased in carpus striatum of rat when treated with ACR (Yousef and El-Demerdash, 2006). As shown in Table 1 and Fig. 2, in this study, we observed the similar result in case LPO and GSH as stated by Mehhri et al. (2015) and Yousef and El-Demerdash (2006).
GSTs specific activity with the substrate CHP is the presence of peroxidase activity that is associated with GST (Dasari et al., 2018b), low level activity of GPx is associated with higher levels of ROS and cellular damage (Chatziagyriou and Dailianis, 2010). Both the peroxidase system and thiol (GSH) status impairment lead to lower rate of peroxidase activity that affects the immune cells and lower survive rate of animal when exposed to a pro-oxidant (Trevisan et al., 2014). In the present study, as shown in Table 1 and Figure 2 & 3, in both liver and kidney the GPx activity with the substrate CHP was significantly decreased (p˂0.05) to 2.79 fold and 2.25 fold, respectively than compared to control i.e. 4.62 fold and 4.19 fold respectively, at 27th day post ingestion. In this study, we observed that similar result as stated by Chatziagyriou and Dailianis (2010), Trevisan et al. (2014) in ACR administered rat.
Usually, GST catalyse the nucleophilic addition of GSH with exogenous (e.g., environmental pollutants and carcinogens) and endogenous (e.g., oxidative stress products) toxic agents which have electrophilic functional groups, hence neutralized, more water soluble compounds are formed and they finally removed from the cell (Coleman et al., 1997). GST activity assay is very important to develop the efficient therapeutics and screening of new anticancer drugs. GST is protecting the cells through detoxification from reactive electrophiles which reduce cells metabolizing ability (Di Pietro et al., 2010). GST polymorphism which increase the defencelessness of the organisms to various xenobitics (Dasari et al., 2018c). The substrate CDNB is the efficient probe for GST activity (Habig et al., 1974). GSTs detoxify that the wide range hazardous substances through transferase activity and GST associated peroxidase activity (Dasari et al., 2017b). In this study, in both liver and kidney as shown in Table 1 and Figure 2 & 3, with the substrate CDNB the GST activity was significantly decreased (p˂0.05) to 3.85 fold and 3.63 fold respectively than control i.e. 5.93 fold and 5.37 fold respectively at 27th day post ingestion. In this study, we observed the similar result as stated by Habig et al. (1974) and Dasari et al. (2017b).
In the present study, ACR administered rat liver and kidney histological changes such as enlarged sinusoid, fragmentation of hepatocyte nuclei, inflammation of Kupffer cells and spreading hepatic contents towards central vein, and tubular thyroidisation, inflammation in distal tubules, distorted glomerulus and proximal tubules fragmentation were observed on 27th day post ingestion as shown in Figure 4 (B) and 5 (B) when compared to control i.e. Figure 4 (A) and 5 (A). Liver degenerative changes like nodular regenerative hyperplasia, perisinusoidal fibrosis, hepatoportal sclerosis and incomplete septal cirrhosis are caused by several factors that include xenobiotic (Hillaire et al., 2002). In kidney, mainly proximal tubule cells are exposed to drugs hence they greatly influenced by toxicity of drugs during the process of concentration and reabsorption through the glomerulus (Perazella, 2005). ACR induced stress enhance ROS that increase lipid peroxidation and oxidation of fatty acids therefore produced lipid peroxyl radicals and lipid hydroxides (Al-Serwia and Ghoneim, 2015), lipid peroxyl radicals are converted as malondialdehyde (MDA) that is a marker for oxidative damage of proteins and lipoproteins, and it is a possible reason for liver injury (Ayala et al., 2014), GSH levels were decreased in ACR treated liver (Park et al., 2002), the large dose of ACR leads to the degeneration and necrosis of hepatic paramchyma in rat (AL Mosaibih, 2013). ACR induced nephrotoxicity which may be related to the generation of free radicals, redox state imbalance, the marked oxidation of membrane lipids and proteins leading to the loss of kidney membrane integrity (Ghorbel et al., 2017). That the nephrotoxicity induced by drug is associated with acute renal damage and chronic kidney diseases (Kim and Moon, 2012). In this study, we observed the similar degenerative changes in rat liver and kidney that administered by ACR.
CONCLUSION
In this study, we observed that the increase of lipid peroxidation and NO levels, and depletion of GSH. Also decrease the GPx and GST enzyme activities and degenerative changes at histological level. The observations suggesting, in rat ACR has induced non-enzymatic and enzymatic perturbations in the cell as well as degeneration of tissue. In conclusion, the ACR in drinking water as well as in aquatic environment cause the serious physiological complications and tissue damage in organisms
ACKNOWLEDGEMENTS
I am thankful to University Grants Commission, New Delhi, India (Grant No: PDFSS-2011-12-SC-AND-3355) for providing Financial Assistance.
CONFLICT OF INTEREST
Authors do not have any potential conflict of interest.
Authors Contribution
B. Meriga has designed work, MS Ganjayi, S. Gonuguntla, SR. Kothapalli and P. Yellanur Konda analyzed data, S. Basha and P. Kutagolla proof read and S. Dasari has carried out experiments and written manuscript.
REFERENCES